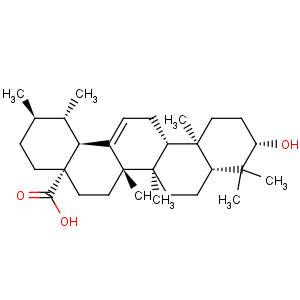
Title: Ursolic Acid
CAS Registry Number: 77-52-1
CAS Name: (3b)-3-Hydroxyurs-12-en-28-oic acid
Synonyms: urson; prunol; micromerol; malol
Molecular Formula: C30H48O3
Molecular Weight: 456.70
Percent Composition: C 78.90%, H 10.59%, O 10.51%
Literature References: In leaves and berries of
Arctostaphylos uva-ursi (L.) Spreng (bearberry), of
Vaccinium macrocarpon Ait. (cranberry),
Rhododendron hymenanthes Makino,
Ericaceae. In the protective wax-like coating of apples, pears, prunes, and other fruits. Isoln from apple peelings: Sando,
J. Biol. Chem. 56, 457 (1923). Structure: Ruzicka
et al., Helv. Chim. Acta 28, 199 (1945); Zurcher
et al., ibid. 37, 2145 (1954). Conversion from a-amyrin: Boar
et al., J. Chem. Soc. C 1970, 678. Chemistry: Mezzetti
et al., Planta Med. 20, 244 (1971).
Properties: Large, lustrous prisms from abs alcohol, fine hair-like needles from dil alcohol, mp 285-288°. [a]D21 +67.5° (c = 1 in
N alc KOH). Soly at 15°: One part dissolves in 88 parts methanol, 178 alcohol (35 boiling alcohol), 140 ether, 388 chloroform, 1675 carbon disulfide. Moderately sol in acetone. Sol in hot glacial acetic acid and in 2% alcoholic NaOH. Insol in water and petr ether.
Melting point: mp 285-288°
Optical Rotation: [a]D21 +67.5° (c = 1 in
N alc KOH)
Derivative Type: Acetate
Molecular Formula: C32H50O4
Molecular Weight: 498.74
Percent Composition: C 77.06%, H 10.10%, O 12.83%
Properties: mp 289-290°. [a]D +62.3° (c = 1.15 in chloroform).
Melting point: mp 289-290°
Optical Rotation: [a]D +62.3° (c = 1.15 in chloroform)
Derivative Type: Methyl ester
Molecular Formula: C31H50O3
Molecular Weight: 470.73
Percent Composition: C 79.10%, H 10.71%, O 10.20%
Properties: mp 171°. [a]D20 +58° (c = 1.2 in pyridine).
Melting point: mp 171°
Optical Rotation: [a]D20 +58° (c = 1.2 in pyridine)
Derivative Type: Methyl ester acetate
Molecular Formula: C33H52O4
Molecular Weight: 512.76
Percent Composition: C 77.30%, H 10.22%, O 12.48%
Properties: mp 246-247°.
Melting point: mp 246-247°
Use: As emulsifying agent in pharmaceuticals, foods.