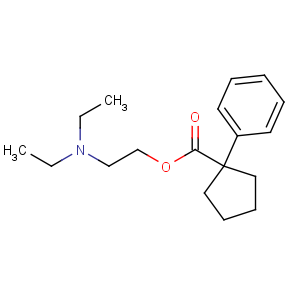
Title: Caramiphen
CAS Registry Number: 77-22-5
CAS Name: 1-Phenylcyclopentanecarboxylic acid 2-(diethylamino)ethyl ester
Synonyms: diethylaminoethyl 1-phenylcyclopentane-1-carboxylate
Molecular Formula: C18H27NO2
Molecular Weight: 289.41
Percent Composition: C 74.70%, H 9.40%, N 4.84%, O 11.06%
Literature References: Anticholinergic. Prepn:
CH 234452; H. Martin, F. H?fliger,
US 2404588 (1945, 1946 both to Geigy). Prepn of the ethanedisulfonate:
CH 272708 (1951 to Geigy),
C.A. 46, 4563i (1952);
Chem. Zentralbl. 1952, 1571. Pharmacology and toxicology of the hydrochloride: C. P. Kraatz
et al., J. Pharmacol. Exp. Ther. 96, 42 (1949); of the ethanedisulfonate: J. J. Toner, E. Macko,
ibid. 106, 246 (1952). GLC determn in blood: P. Levandoski, T. Flanagan,
J. Pharm. Sci. 69, 1353 (1980). Mechanism of antitussive action: E. F. Domino
et al., J. Pharmacol. Exp. Ther. 233, 249 (1985).
Properties: bp0.07 112-115°.
Boiling point: bp0.07 112-115°
Derivative Type: Ethanedisulfonate
CAS Registry Number: 125-86-0
Molecular Formula: (C18H27NO2)2.C2H6O6S2
Molecular Weight: 769.02
Percent Composition: C 59.35%, H 7.86%, N 3.64%, O 20.80%, S 8.34%
Properties: Crystals from acetone, mp 115-116°. More sol in water than the hydrochloride. Sol in alc, pharmaceutical syrups. Mixture with phenylpropanolamine hydrochloride,
Tuss-Ornade (SKB) .
Melting point: mp 115-116°
Derivative Type: Hydrochloride
CAS Registry Number: 125-85-9
Molecular Formula: C18H27NO2.HCl
Molecular Weight: 325.87
Percent Composition: C 66.34%, H 8.66%, N 4.30%, O 9.82%, Cl 10.88%
Properties: Crystals, mp 145-146°. Sol in alc; slightly sol in water. LD50 i.p. in rats: 209 mg/kg (Kraatz).
Melting point: mp 145-146°
Toxicity data: LD50 i.p. in rats: 209 mg/kg (Kraatz)
Therap-Cat: Antitussive.
Keywords: Antitussive.