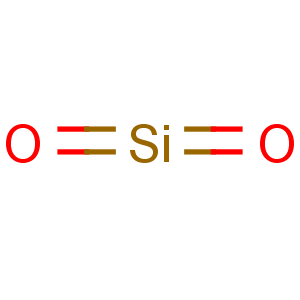Title: Silicon Dioxide
CAS Registry Number: 7631-86-9
CAS Name: Silica
Synonyms: silicic anhydride
Molecular Formula: O2Si
Molecular Weight: 60.08
Percent Composition: O 53.26%, Si 46.75%
Line Formula: SiO2
Literature References: Occurs in nature as
agate,
amethyst,
chalcedony,
cristobalite,
flint,
quartz,
sand,
tridymite.
Reviews: Kirk-Othmer Encyclopedia of Chemical Technology vol. 18 (Interscience, New York, 2nd ed., 1969) pp 46-111; Rochow in
Comprehensive Inorganic Chemistry vol. 1, J. C. Bailar, Jr.
et al., Eds. (Pergamon Press, Oxford, 1973) pp 1388-1402. Toxicology: L. T. Fairhall,
Industrial Toxicology (Hafner, New York, 1969) pp 105-107.
Properties: Transparent, tasteless crystals, or amorphous powder. d (amorphous) 2.2. d0 (quartz) 2.65. Melts to a glass. Silica has the lowest coefficient of expansion by heat of any known substance. It is practically insol in water or acids, except hydrofluoric acid in which it readily dissolves forming the gas silicon tetrafluoride; it is also slowly attacked by heating with concd phosphoric acid. The crystallized forms of silica are scarcely attacked by alkalies, while the amorphous is sol, especially when finely divided.
See also Infusorial Earth.
Density: d (amorphous) 2.2; d0 (quartz) 2.65
CAUTION: Potential symptom of overexposure to amorphous silica are eye irritation and pneumoconiosis. Potential symptoms of overexposure to crystalline silica as respirable dust are cough, dyspnea, wheezing; decreased pulmonary function, progressive respiratory symptoms (silicosis).
See NIOSH Pocket Guide to Chemical Hazards (DHHS/NIOSH 97-140, 1997) p 276-279. Crystalline silica (respirable size), primarily quartz dusts occurring in industrial and occupational settings, is listed as a known human carcinogen:
Report on Carcinogens, Eleventh Edition (PB2005-104914, 2004) p III-231.
Use: Manuf glass, water glass, refractories, abrasives, ceramics, enamels; decolorizing and purifying oils, petroleum products, etc.; in scouring- and grinding-compounds, ferrosilicon, molds for castings; as anticaking and defoaming agent.