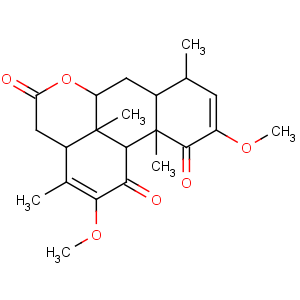
Title: Quassin
CAS Registry Number: 76-78-8
CAS Name: 2,12-Dimethoxypicrasa-2,12-diene-1,11,16-trione
Synonyms: 3ab,6ab,7,7aa,8,11a,11ba,11c-octahydro-2,10-dimethoxy-3,8a,11ab,11cb-tetramethylphenanthro[10,1-
bc]pyran-1,5,11(4
H)-trione; nigakilactone D
Molecular Formula: C22H28O6
Molecular Weight: 388.45
Percent Composition: C 68.02%, H 7.27%, O 24.71%
Literature References: One of the bitter constituents of the wood of
Quassia amara L.,
Simaroubaceae known in commerce as Surinam quassia. Obtained by the resolution of the mixture of bitter constituents of quassia wood: E. P. Clark,
J. Am. Chem. Soc. 59, 927 (1937); London
et al., J. Chem. Soc. 1950, 3431. Structure: Valenta
et al., Tetrahedron Lett. no.
20, 25 (1960); Carman, Ward,
ibid. 1961, 317; Valenta
et al., Tetrahedron 15, 100 (1961). Stereochemistry: Valenta
et al., ibid. 18, 1433 (1962). Identity with nigakilactone D: Murae
et al., ibid. 27, 1545 (1971). Synthetic approach: Stojanac
et al., Can. J. Chem. 53, 619 (1975); P. A. Grieco
et al., Tetrahedron Lett. 21, 1619 (1980). Total synthesis of
dl-form:
eidem, J. Am. Chem. Soc. 102, 7586 (1980).
Properties: Very bitter rectangular plates from dilute methanol, mp 222°. [a]D20 +34.5° (c = 5.09 in CHCl3). uv max: ~255 nm (e ~11,650). Sol in benzene, alc, acetone, chloroform, pyridine, acetic acid, hot ethyl acetate. Sparingly sol in ether, petr ether. Bitterness threshold 1:60,000.
Melting point: mp 222°
Optical Rotation: [a]D20 +34.5° (c = 5.09 in CHCl3)
Absorption maximum: uv max: ~255 nm (e ~11,650)