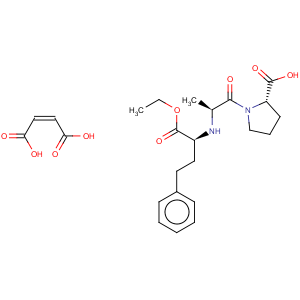
Title: Enalapril
CAS Registry Number: 75847-73-3
CAS Name: N-[(1
S)-1-(Ethoxycarbonyl)-3-phenylpropyl]-L-alanyl-L-proline
Synonyms: 1-[
N-[(
S)-1-carboxy-3-phenylpropyl]-L-alanyl]-L-proline 1¢-ethyl ester
Molecular Formula: C20H28N2O5
Molecular Weight: 376.45
Percent Composition: C 63.81%, H 7.50%, N 7.44%, O 21.25%
Literature References: Angiotensin-converting enzyme (ACE) inhibitor; de-esterified
in vivo to its active diacid metabolite, enalaprilat,
q.v. Prepn: A. A. Patchett
et al., Nature 288, 280 (1980);
eidem, EP 12401; E. E. Harris
et al., US 4374829 (1980, 1983 both to Merck & Co.). Pharmacology: D. M. Gross
et al., J. Pharmacol. Exp. Ther. 216, 552 (1981); C. S. Sweet
et al., ibid. 558. Bioavailability and metabolism: E. H. Ulm,
Drug Metab. Rev. 14, 99 (1983). Comprehensive description: D. P. Ip, G. S. Brenner,
Anal. Profiles Drug Subs. 16, 207-243 (1987). Clinical trial in congestive heart failure: Consensus Trial Study Group,
N. Engl. J. Med. 316, 1429 (1987). Review of clinical experience in hypertension: H. J. Gomez
et al., J. Cardiovasc. Pharmacol. 15, Suppl. 3, S26-S29 (1990); of clinical pharmacokinetics: R. J. MacFadyen
et al., Clin. Pharmacokinet. 25, 274-282 (1993); of combination with hydrochlorothiazide: P. L. Malini,
Adv. Ther. 10, 253-262 (1993).
Derivative Type: Maleate
CAS Registry Number: 76095-16-4
Manufacturers' Codes: MK-421
Trademarks: Amprace (Merck & Co.); Cardiovet (Intervet); Enacard (Merial); Enapren (Merck & Co.); Glioten (Armstrong); Hipoartel (Ipsen); Innovace (Merck & Co.); Naprilene (Sigma-Tau); Pres (Boehringer, Ing.); Renitec (Merck & Co.); Reniten (Merck & Co.); Renivace (Merck & Co.); Vasotec (Merck & Co.); Xanef (Merck & Co.)
Molecular Formula: C20H28N2O5.C4H4O4
Molecular Weight: 492.52
Percent Composition: C 58.53%, H 6.55%, N 5.69%, O 29.24%
Properties: White to off-white crystalline powder, mp 143-144.5°. Soly (g/ml): water 0.025; alcohol 0.08; methanol 0.20. [a]D25 -42.2° (c = 1 in methanol). pH (1% water) 2.6. pKa1 3.0; pKa2 (25°) 5.4.
Melting point: mp 143-144.5°
pKa: pKa1 3.0; pKa2 (25°) 5.4
Optical Rotation: [a]D25 -42.2° (c = 1 in methanol)
Derivative Type: Mixture of maleate with hydrochlorothiazide
Trademarks: Acesistem (Sigma-Tau); Co-Renitec (Merck & Co.); Enaloc (Leiras); Innozide (Merck & Co.); Lotrial D (Roemmers); Renacor (Merck & Co.); Vaseretic (Merck & Co.); Vasoretic (Merck & Co.); Xynertec (Merck & Co.)
Therap-Cat: Antihypertensive.
Therap-Cat-Vet: In treatment of heart failure in dogs.
Keywords: ACE-Inhibitor; Antihypertensive; N-Carboxyalkyl (peptide/lactam) Derivatives.