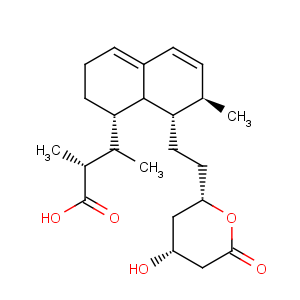
Title: Mevastatin
CAS Registry Number: 73573-88-3
CAS Name: (2
S)-2-Methylbutanoic acid (1
S,7
S,8
S,8a
R)-1,2,3,7,8,8a-hexahydro-7-methyl-8-[2-[(2
R,4
R)-tetrahydro-4-hydroxy-6-oxo-2
H-pyran-2-yl]ethyl]-1-naphthalenyl ester
Synonyms: 7-[1,2,6,7,8,8a-hexahydro-2-methyl-8-(methylbutyryloxy)naphthyl]-3-hydroxyheptan-5-olide; 2b-methyl-8a-(2-methyl-1-oxobutoxy)mevinic acid lactone; compactin; 6-demethylmevinolin
Manufacturers' Codes: CS-500; ML-236 B
Molecular Formula: C23H34O5
Molecular Weight: 390.51
Percent Composition: C 70.74%, H 8.78%, O 20.49%
Literature References: Fungal metabolite which is a potent inhibitor of HMG-CoA reductase, the rate controlling enzyme in cholesterol biosynthesis. Isoln from
Penicillium citrinum: A. Endo
et al., DE 2524355 corresp to
US 3983140 (1975, 1976 to Sankyo). Isoln from
P. brevicompactum, crystal and molecular structure: A. G. Brown
et al., J. Chem. Soc. Perkin Trans. 1 1976, 1165. Inhibition of HMG-CoA reductase activity: A. Endo
et al., FEBS Lett. 72, 323 (1976); M. S. Brown
et al., J. Biol. Chem. 253, 1121 (1978). Therapeutic effects in primary hypercholesterolemia: A. Yamamoto
et al., Atherosclerosis 35, 259 (1980). Total synthesis: N. Y. Wang
et al., J. Am. Chem. Soc. 103, 6538 (1981); M. Hirama, M. Uei,
ibid. 104, 4251 (1982); N. N. Girotra, N. L. Wendler,
Tetrahedron Lett. 23, 5501 (1982); C.-T. Hsu
et al., J. Am. Chem. Soc. 105, 593 (1983); P. A. Grieco
et al., ibid. 1403; D. L. J. Clive
et al., J. Am. Chem. Soc. 110, 6914 (1988). Review of syntheses: T. Rosen, C. H. Heathcock,
Tetrahedron 42, 4909-4951 (1986). Review of mevastatin and related compounds: A. Endo,
J. Med. Chem. 28, 401-405 (1985).
Properties: Crystals from aq ethanol, mp 152°. [a]D22 +283° (c = 0.48 in acetone). uv max: 230, 237, 246 nm (log e 4.28, 4.30, 4.11).
Melting point: mp 152°
Optical Rotation: [a]D22 +283° (c = 0.48 in acetone)
Absorption maximum: uv max: 230, 237, 246 nm (log e 4.28, 4.30, 4.11)