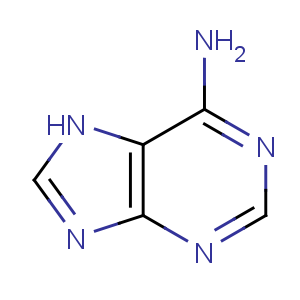
Title: Adenine
CAS Registry Number: 73-24-5
CAS Name: 1
H-Purin-6-amine
Synonyms: 6-aminopurine; 6-amino-1
H-purine; 6-amino-3
H-purine; 6-amino-9
H-purine; 1,6-dihydro-6-iminopurine; 3,6-dihydro-6-iminopurine
Trademarks: Leuco-4
Molecular Formula: C5H5N5
Molecular Weight: 135.13
Percent Composition: C 44.44%, H 3.73%, N 51.83%
Literature References: Also referred to as
vitamin B4: Lecoq,
Int. Z. Vitaminforsch. 27, 291 (1957). Widespread throughout animal and plant tissues combined with niacinamide, D-ribose, and phosphoric acids; a constituent of nucleic acids and coenzymes, such as codehydrase I and II, adenylic acid, coalaninedehydrase. Isoln from bovine pancreas: Kossel,
Ber. 18, 79, 1928 (1885). Syntheses: Fischer,
ibid. 30, 2226 (1897); Traube,
Ann. 331, 64 (1904); Hoffer,
Jubilee Vol. Emil Barell 1946, 428-434; Taylor
et al., Ciba Found. Symp. Chem. Biol. Purines 1957, 20,
C.A. 53, 6238b (1959); Bredereck
et al., Angew. Chem. 71, 524 (1959); Morita
et al., Chem. Ind. (London) 1968, 1117; Sekiya, Suzuki,
Chem. Pharm. Bull. 20, 209 (1972); N. J. Kos
et al., J. Org. Chem. 44, 3140 (1979). Toxicity study: Philips
et al., J. Pharmacol. Exp. Ther. 104, 20 (1952).
Review: Ts'o, "Bases, Nucleosides and Nucleotides" in
Basic Principles in Nucleic Acid Chemistry vol. 1, P. O. P. Ts'o, Ed. (Academic Press, New York, 1974) pp 453-584.
Properties: Trihydrate, orthorhombic needles. Anhydr at 110°, dec 360-365°, subl 220°. uv max (pH 7.0): 207, 260.5 nm (e ′ 10-3 23.2, 13.4). One gram of anhydr compd dissolves in 2000 ml water, 40 ml boiling water; slightly sol in alc. Practically insol in ether, CHCl3. Aq solns are neutral. Combines with acids and bases. LD50 orally in rats: 745 mg/kg (Philips).
Absorption maximum: uv max (pH 7.0): 207, 260.5 nm (e ′ 10-3 23.2, 13.4)
Toxicity data: LD50 orally in rats: 745 mg/kg (Philips)
Derivative Type: Hydrochloride hemihydrate
Properties: Monoclinic prisms. One gram dissolves in 42 ml water.
Derivative Type: Sulfate dihydrate
Properties: Crystals. One gram dissolves in 150 ml water; slightly sol in alc.
Use: In microbial determination of niacin; in research on heredity, virus diseases, and cancer.