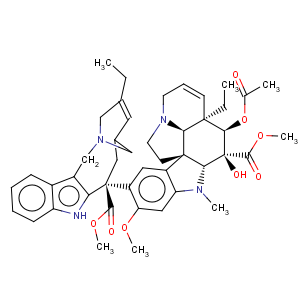
Title: Vinorelbine
CAS Registry Number: 71486-22-1
CAS Name: (2b,3b,4b,5a,12
R,19a)-4-(Acetyloxy)-6,7-didehydro-15-[(2
R,6
R,8
S)-4-ethyl-1,3,6,7,8,9-hexahydro-8-(methoxycarbonyl)-2,6-methano-2
H-azecino[4,3-
b]indol-8-yl]-3-hydroxy-16-methoxy-1-methylaspidospermidine-3-carboxylic acid methyl ester
Synonyms: 3¢,4¢-didehydro-4¢-deoxy-
C¢-norvincaleukoblastine; nor-5¢-anhydrovinblastine; NVB
Manufacturers' Codes: KW-2307
Molecular Formula: C45H54N4O8
Molecular Weight: 778.93
Percent Composition: C 69.39%, H 6.99%, N 7.19%, O 16.43%
Literature References: Semi-synthetic
Vinca alkaloid; structurally related to vinblastine,
q.v. Prepn:
JP Kokai 80 31096; N. Langlois
et al., US 4307100 (1980, 1981 both to Agence Nat. Valorisation Recherche); P. Mangeney
et al., Tetrahedron 35, 2175 (1979). Pharmacology: G. Mathé, P. Reizenstein,
Cancer Lett. 27, 285 (1985). HPLC determn in biological fluids: F. Jehl
et al., J. Chromatogr. 525, 225 (1990). Veterinary trial in dogs with spontaneous neoplasia: V. J. Poirier
et al., J. Vet. Intern. Med. 18, 536 (2004). Symposium:
Semin. Oncol. 16, Suppl. 4, 1-45 (1989). Review of early clinical development: M. Marty
et al., Nouv. Rev. Fr. Hematol. 31, 77-84 (1989); of clinical pharmacokinetics: D. Leveque, F. Jehl,
Clin. Pharmacokinet. 31, 184-197 (1996); of use in advanced non-small cell lung cancer: M. P. Curran, G. L. Plosker,
Drugs Aging 19, 695-721 (2002).
Properties: Residue. [a]D20 +52.4° (c = 0.3 in CHCl3). uv max (ethanol): 215, 268, 282, 293, 310 nm (e 3700, 11000, 9500, 7600, 4400). Partition coefficient (octanol/buffer pH 7.2): 16. LD50 in mice (mg/m2): 72 i.v.; 78 orally (Mathé, Reizenstein).
Optical Rotation: [a]D20 +52.4° (c = 0.3 in CHCl3)
Log P: Partition coefficient (octanol/buffer pH 7.2): 16
Absorption maximum: uv max (ethanol): 215, 268, 282, 293, 310 nm (e 3700, 11000, 9500, 7600, 4400)
Toxicity data: LD50 in mice (mg/m2): 72 i.v.; 78 orally (Mathé, Reizenstein)
Derivative Type: Ditartrate
CAS Registry Number: 125317-39-7
Trademarks: Eunades (Pfizer); Navelbine (Fabre)
Molecular Formula: C45H54N4O8.2C4H6O6
Molecular Weight: 1079.11
Percent Composition: C 58.99%, H 6.16%, N 5.19%, O 29.65%
Properties: Yellow-white amorphous powder. Sol in water and ethanol.
Therap-Cat: Antineoplastic.
Therap-Cat-Vet: Antineoplastic.
Keywords: Antineoplastic; Alkaloids/Natural Products; Vinca Alkaloids.