Title: Mecoprop
CAS Registry Number: 7085-19-0
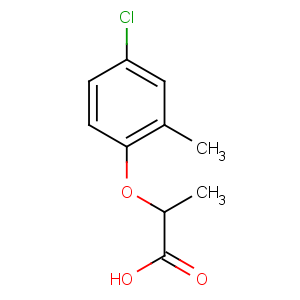
CAS Name: 2-(4-Chloro-2-methylphenoxy)propanoic acid
Synonyms: (±)-2-[(4-chloro-
o-tolyl)oxy]propionic acid; mechlorprop; MCPP; CMPP
Manufacturers' Codes: RD-4593
Trademarks: Astix CMPP (Agrotec); Iso-Cornox (FBC); Compitox (M & B); Compitox Plus (M & B); Proponex-Plus (Shell)
Molecular Formula: C10H11ClO3
Molecular Weight: 214.65
Percent Composition: C 55.95%, H 5.17%, Cl 16.52%, O 22.36%
Literature References: Prepn: M. E. Synerholm, P. W. Zimmerman,
Contrib. Boyce Thompson Inst. 14, 91 (1945). Studies on plant growth regulation: C. H. Fawcett
et al., Ann. Appl. Biol. 40, 231 (1953); and comparison of enantiomers: M. Matell,
Kungl. Lantbruks-Hogsk. Ann. 20, 207 (1953); B. Aberg,
ibid. 241. GLC determn: H. G. Higson, D. Butler,
Analyst 85, 657 (1960). Crystal structure: G. Smith
et al., Acta Crystallogr. B36, 992 (1980). Herbicidal activity: G. B. Lush,
Proc. 3rd Br. Weed Control Conf. 625 (1956); E. L. Leafe,
ibid. 633; B. Wallgren,
Weeds Weed Contr. 24th Swedish Weed Conf. 30 (1983); of (+)-enantiomer: J. Toll,
Weeds Weed Contr. 28th Swedish Weed Conf. 100 (1987). Degradation in soils: L. Lindholm
et al., Acta Agric. Scand. 32, 429 (1982); A. E. Smith,
Bull. Environ. Contam. Toxicol. 34, 656 (1985). Toxicological studies: M. R. Gurd
et al., Food Cosmet. Toxicol. 3, 883 (1965); H. G. Verschuuren
et al., Toxicology 3, 349 (1975); R. Roll, G. Matthiaschk,
Arzneim.-Forsch. 33, 1479 (1983). EC-GLC determn in tissues and biological fluids: J. De Beer
et al., Vet. Hum. Toxicol. 21, Suppl., 172 (1979). HPLC resolution of enantiomers: B. Blessington
et al., J. Chromatogr. 396, 177 (1987).
Properties: Solid, mp 93-94°. LD50 in rats (mg/kg): 1210 orally, 402 i.p. (Verschuuren).
Melting point: mp 93-94°
Toxicity data: LD50 in rats (mg/kg): 1210 orally, 402 i.p. (Verschuuren)
Derivative Type: (+)-Form
Synonyms: Mecoprop-P
Trademarks: Duplosan KV (BASF)
Properties: Solid, mp 95-96°. [a]D25 +19° (alcohol).
Melting point: mp 95-96°
Optical Rotation: [a]D25 +19° (alcohol)
Derivative Type: Sodium salt
Molecular Formula: C10H11ClNaO3
Molecular Weight: 237.64
Percent Composition: C 50.54%, H 4.67%, Cl 14.92%, Na 9.67%, O 20.20%
Properties: LD50 i.p. in rats, mice: 500, 600 mg/kg; orally in mice: 650 mg/kg (Gurd).
Toxicity data: LD50 i.p. in rats, mice: 500, 600 mg/kg; orally in mice: 650 mg/kg (Gurd)
Derivative Type: Diethylamine salt
Trademarks: Mecopar
Molecular Formula: C14H22ClNO3
Molecular Weight: 287.78
Percent Composition: C 58.43%, H 7.71%, Cl 12.32%, N 4.87%, O 16.68%
Properties: LD50 in rats, mice (mg/kg): 1060 ±120, 600 ±35 orally; 350, 400 i.p. (Gurd).
Toxicity data: LD50 in rats, mice (mg/kg): 1060 ±120, 600 ±35 orally; 350, 400 i.p. (Gurd)
Derivative Type: Potassium salt
Trademarks: Mecomec (PBI/Gordon); Hedonal MCPP (Bayer)
Molecular Formula: C10H11ClKO3
Molecular Weight: 253.74
Percent Composition: C 47.33%, H 4.37%, Cl 13.97%, K 15.41%, O 18.92%
Use: Herbicide.