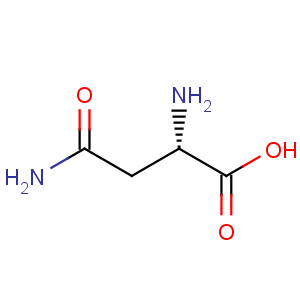
Title: Asparagine
CAS Registry Number: 70-47-3
CAS Name: L-Asparagine
Synonyms: Asn; N; L-b-asparagine; a-aminosuccinamic acid; aspartic acid b-amide; altheine; asparamide; agedoite; (
S)-2,4-diamino-4-oxobutanoic acid
Molecular Formula: C4H8N2O3
Molecular Weight: 132.12
Percent Composition: C 36.36%, H 6.10%, N 21.20%, O 36.33%
Literature References: Non-essential amino acid for human development. First amino acid noted in natural sources, it was named for the asparagus juice from which it was isolated: L. N. Vauquelin, P. J. Robiquet,
Ann. Chim. 57, 88 (1806). Due to ease of conversion to aspartic acid,
q.v., it was not isolated from protein until 1932: M. Damodoran,
Biochem. J. 26, 235 (1932). Early chemistry and biochemistry:
Amino Acids and Proteins, D. M. Greenberg, Ed. (Charles C. Thomas, Springfield, IL, 1951) 950 pp,
passim; J. P. Greenstein, M. Winitz,
Chemistry of the Amino Acids vols. 1-3 (John Wiley & Sons, Inc., New York, 1961) pp. 1856-1878,
passim. As attachment point for saccharides: A. J. Mencke
et al., Methods Enzymol. 138, 409 (1987). Colorimetric assay: S. Sheng
et al., Anal. Biochem. 211, 242 (1993). Proposed formation of acrylamide,
q.v., in cooking: D. S. Mottram
et al.,
Nature 419, 448 (2002); R. H. Stadler
et al.,
ibid. 449. Review of metabolism: D. A. Cooney, R. E. Handschumacher,
Annu. Rev. Pharmacol. 10, 421-440 (1970); in plants: K. A. Sieciechowicz
et al., Phytochemistry 27, 663-671 (1988). Review of deamidation in proteins: R. Bischoff, H. V. J. Kolbe,
J. Chromatogr. B 662, 261-278 (1994).
Properties: Orthorhombic bisphenoidal crystals, mp 234-235° (bath preheated to 226°). d415 1.543. Acid to litmus. pK1 2.02; pK2 8.80. [a]D20 -5.30° (c = 1.41); [a]D20 +34.26° (c = 2.24 in 3.4
N HCl); [a]D20 -6.35° (c = 11.23 in 2.5
N NaOH). Practically insol in methanol, ethanol, ether, benzene. Sol in acids, alkalies.
Melting point: mp 234-235° (bath preheated to 226°)
pKa: pK1 2.02; pK2 8.80
Optical Rotation: [a]D20 -5.30° (c = 1.41); [a]D20 +34.26° (c = 2.24 in 3.4
N HCl); [a]D20 -6.35° (c = 11.23 in 2.5
N NaOH)
Density: d415 1.543
Derivative Type: D-Form
CAS Registry Number: 2058-58-4
Literature References: In peptidoglycans of bacterial cell walls: D. A. Reaveley, R. E. Burge,
Adv. Microb. Physiol. 7, 1-81 (1972).
Derivative Type: D-Form monohydrate
Properties: Crystals, mp 215°. [a]D20 +5.41° (c = 1.3).
Melting point: mp 215°
Optical Rotation: [a]D20 +5.41° (c = 1.3)