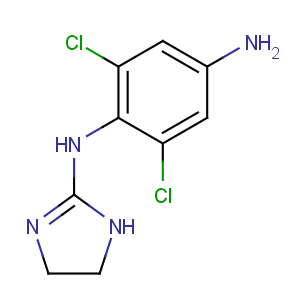
Title: Apraclonidine
CAS Registry Number: 66711-21-5
CAS Name: 2,6-Dichloro-
N1-(4,5-dihydro-1
H-imidazol-2-yl)-1,4-benzenediamine
Synonyms: 2,6-dichloro-
N¢-2-imidazolidinylidene-1,4-benzenediamine; 2-[(4-amino-2,6-dichlorophenyl)imino]imidazolidine;
p-aminoclonidine; aplonidine
Manufacturers' Codes: NC-14
Molecular Formula: C9H10Cl2N4
Molecular Weight: 245.11
Percent Composition: C 44.10%, H 4.11%, Cl 28.93%, N 22.86%
Literature References: a2-Adrenergic agonist; structural analog of clonidine,
q.v. Synthesis: B. Rouot, G. LeClerc,
Bull. Soc. Chim. Fr. Pt. 2, 520 (1979). Prepn (not claimed) and use in treatment of intraocular pressure: B. M. York, Jr.,
US 4517199 (1985 to Alcon). Pharmacology: B. Rouot
et al., C.R. Seances Acad. Sci. Ser. D 286, 909 (1978). Receptor binding studies: D. C. U'Prichard,
Prog. Clin. Biol. Res. 71, 53 (1981). D. C. Stump, D. E. MacFarlane,
J. Lab. Clin. Med. 102, 779 (1983). Clinical pharmacology: D. A. Abrams
et al., Arch. Ophthalmol. 105, 1205 (1987). Clinical evaluation in treatment of intraocular pressure: A. L. Robin
et al., ibid. 1208; as additive to maximally tolerated medical therapy in glaucoma: A. L. Robin
et al., Am. J. Ophthalmol. 120, 423 (1995).
Properties: Solid, mp >230°.
Melting point: mp >230°
Derivative Type: Hydrochloride
CAS Registry Number: 73218-79-8
Manufacturers' Codes: ALO-2145
Trademarks: Iopidine (Alcon)
Molecular Formula: C9H10Cl2N4.HCl
Molecular Weight: 281.57
Percent Composition: C 38.39%, H 3.94%, Cl 37.77%, N 19.90%
Derivative Type: Dihydrochloride
CAS Registry Number: 73217-88-6
Molecular Formula: C9H10Cl2N4.2HCl
Molecular Weight: 318.03
Percent Composition: C 33.99%, H 3.80%, Cl 44.59%, N 17.62%
Properties: uv max (ethanol): 254, 304 nm (e 1800, 2500).
Absorption maximum: uv max (ethanol): 254, 304 nm (e 1800, 2500)
Therap-Cat: Treatment of post-surgical elevated intraocular pressure.
Keywords: a-Adrenergic Agonist; Antiglaucoma.