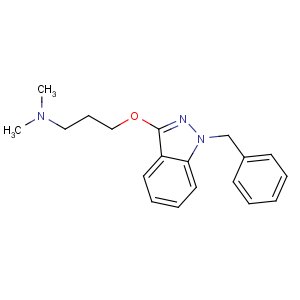
Title: Benzydamine
CAS Registry Number: 642-72-8
CAS Name: N,N-Dimethyl-3-[[1-(phenylmethyl)-1
H-indazol-3-yl]oxy]-1-propanamine
Synonyms: 1-benzyl-3-[3-(dimethylamino)propoxy]-1
H-indazole; 1-benzyl-1
H-indazol-3-yl 3-(dimethylamino)propyl ether; benzindamine
Molecular Formula: C19H23N3O
Molecular Weight: 309.41
Percent Composition: C 73.75%, H 7.49%, N 13.58%, O 5.17%
Literature References: Prepn:
FR 1382855; Palazzo,
US 3318905 (1964, 1967 both to Angelini Francesco); Palazzo
et al., J. Med. Chem. 9, 38 (1966). Pharmacology: Lisciani
et al., Eur. J. Pharmacol. 3, 157 (1968). Metabolism: Catanese
et al., Arzneim.-Forsch. 16, 1354 (1966); Kataoka
et al., Chem. Pharm. Bull. 19, 1511 (1971). Toxicology: B. Silvestrini
et al., Toxicol. Appl. Pharmacol. 10, 148 (1967). Series of articles on pharmacology:
Arzneim.-Forsch. 37, 587-646 (1987).
Properties: bp0.05 160°.
Boiling point: bp0.05 160°
Derivative Type: Hydrochloride
CAS Registry Number: 132-69-4
Trademarks: Afloben (Esseti); Andolex (3M Pharma); Benzyrin (Yoshitomi); Difflam (3M Pharma); Enzamin (Kowa); Imotryl (Cassenne); Opalgyne (Innoth?a); Riripen (Daiichi); Salyzoron (Hishiyama); Saniflor (Esseti); Tamas (Schering); Tantum (Angelini); Verax (Tosi)
Molecular Formula: C19H23N3O.HCl
Molecular Weight: 345.87
Percent Composition: C 65.98%, H 6.99%, N 12.15%, O 4.63%, Cl 10.25%
Properties: Crystals, mp 160°. uv max: 306 nm (E1%1cm 160). Very sol in water; rather sol in ethanol, chloroform,
n-butanol. LD50 in mice, rats (mg/kg): 110, 100 i.p.; 515, 1050 orally (Silvestrini).
Melting point: mp 160°
Absorption maximum: uv max: 306 nm (E1%1cm 160)
Toxicity data: LD50 in mice, rats (mg/kg): 110, 100 i.p.; 515, 1050 orally (Silvestrini)
Therap-Cat: Analgesic; anti-inflammatory; antipyretic.
Therap-Cat-Vet: Anti-inflammatory.
Keywords: Analgesic (Non-Narcotic); Anti-inflammatory (Nonsteroidal); Antipyretic.