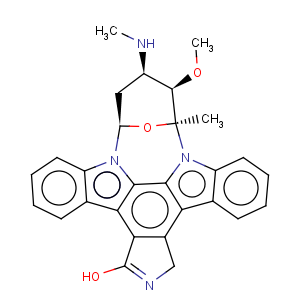
Title: Staurosporine
CAS Registry Number: 62996-74-1
CAS Name: (9
S,10
R,11
R,13
R)- 2,3,10,11,12,13-Hexahydro-10-methoxy-9-methyl-11-(methylamino)-9,13-epoxy-1
H,9
H-diindolo[1,2,3-
gh:3¢,2¢,1¢-
lm]pyrrolo[3,4-
j][1,7]benzodiazonin-1-one
Manufacturers' Codes: AM-2282; CGP-39360
Molecular Formula: C28H26N4O3
Molecular Weight: 466.53
Percent Composition: C 72.09%, H 5.62%, N 12.01%, O 10.29%
Literature References: Protein kinase C inhibitor; alkaloid isolated from
Streptomyces staurosporeus. Isoln: S. Omura
et al., J. Antibiot. 30, 275 (1977). Crystal and molecular structure: A. Furusaki
et al., J. Chem. Soc. Chem. Commun. 1978, 800;
eidem, Bull. Chem. Soc. Jpn. 55, 3681 (1982). Corrected stereochemistry: N. Funato
et al., Tetrahedron Lett. 35, 1251 (1994). Total synthesis: J. T. Link
et al., J. Am. Chem. Soc. 117, 552 (1995);
idem et al., ibid. 118, 2825 (1996). Biosynthetic studies: D. Meksuriyen, G. A. Cordell,
J. Nat. Prod. 51, 884, 893 (1988); S.-W. Yang
et al., ibid. 62 1551 (1999). HPLC determn in blood and pharmacokinetics in rats: L. R. Gurley
et al., J. Chromatogr. B 712, 211 (1998). Inhibition of protein kinase C: T. Tamaoki
et al., Biochem. Biophys. Res. Commun. 135, 397 (1986); of other protein kinases: U. T. Rüegg, G. M. Burgess,
Trends Pharmacol. Sci. 10, 218 (1989). Induction of apoptosis: E. Falcieri
et al., Biochem. Biophys. Res. Commun. 193, 19 (1993); R. Bertrand
et al., Exp. Cell Res. 211, 314 (1994); of tyrosine phosphorylation: D. Rasouly, P. Lazarovici,
Eur. J. Pharmacol. 269, 255 (1994).
Properties: Pale yellow needles from chloroform-methanol as the methanol solvate, mp 270° (dec) (Omura). Also reported as yellow crystals from methanol, mp 288-291° (Meksuriyen, Cordell). [a]D25 +35.0° (c = 1 in methanol); [a]D22 +56.1° (c = 0.14 in methanol). uv max (methanol): 241.0, 266.0, 292.5, 321.5, 335.0, 355.0, 372.5 nm (log e 4.25, 4.26, 4.53, 3.88, 3.96, 3.81, 3.85). Sol in DMSO, DMF. Slightly sol in chloroform, methanol.
Melting point: mp 270° (dec); mp 288-291° (Meksuriyen, Cordell)
Optical Rotation: [a]D25 +35.0° (c = 1 in methanol); [a]D22 +56.1° (c = 0.14 in methanol)
Absorption maximum: uv max (methanol): 241.0, 266.0, 292.5, 321.5, 335.0, 355.0, 372.5 nm (log e 4.25, 4.26, 4.53, 3.88, 3.96, 3.81, 3.85)
Derivative Type: Hydrochloride
Molecular Formula: C28H26N4O3.HCl
Molecular Weight: 502.99
Percent Composition: C 66.86%, H 5.41%, N 11.14%, O 9.54%, Cl 7.05%
Properties: LD50 in mice (mg/kg): 6.6 i.p. (Omura).
Toxicity data: LD50 in mice (mg/kg): 6.6 i.p. (Omura)
Use: Pharmacological tool to study signal transduction pathways, tyrosine phosphorylation and to induce apoptosis.