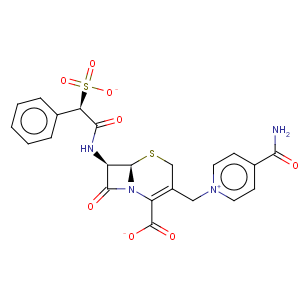
Title: Cefsulodin
CAS Registry Number: 62587-73-9
CAS Name: 4-(Aminocarbonyl)-1-[[(6
R,7
R)-2-carboxy-8-oxo-7-[[(2
R)-phenylsulfoacetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]pyridinium inner salt
Synonyms: 7-(a-sulphophenylacetamido)-3-(4¢-carbamoylpyridinium)methyl-3-cephem-4-carboxylic acid
Molecular Formula: C22H20N4O8S2
Molecular Weight: 532.55
Percent Composition: C 49.62%, H 3.79%, N 10.52%, O 24.03%, S 12.04%
Literature References: Third generation cephalosporin antibiotic. Prepn: S. Morimoto
et al., DE 2234280;
eidem, US 4065619 (1973, 1977 both to Takeda). Prepn and separation of isomers: H. Nomura
et al., J. Med. Chem. 17, 1312 (1974).
In vitro and
in vivo antibacterial activity: K. Tsuchiya
et al., Antimicrob. Agents Chemother. 13, 137 (1978). Absorption, distribution, excretion in mice, rats, dogs:
eidem, J. Antibiot. 31, 593 (1978). Activity and susceptibility to b-lactamases: A. King
et al., Antimicrob. Agents Chemother. 17, 165 (1980).
In vitro comparison with other antibacterials: H. Grimm,
Arzneim.-Forsch. 30, 1478 (1980). Clinical pharmacology: J. Fuellhaas
et al., Curr. Chemother., Proc. 10th Int. Congr. Chemother. (Washington, D.C., 1978)
2, 848-851. Review of activity: H. C. Neu, B. E. Scully,
Rev. Infect. Dis. 6, Suppl. 3, S667-S677 (1984).
Review: A. Bryskier,
Lyon Pharm. 34, 343-355 (1983); D. B. Wright,
Drug Intell. Clin. Pharm. 20, 845-849 (1986).
Derivative Type: Sodium salt
CAS Registry Number: 52152-93-9
Synonyms: Sulcephalosporin
Manufacturers' Codes: Abbott 46811; CGP-7174/E; SCE-129
Trademarks: Cefomonil (TAP); Monaspor (Novartis); Pseudocef (Grñenthal); Pyocefal (Cassenne-Takeda); Takesulin (Takeda); Tilmapor (Novartis); Ulfaret (Takeda)
Molecular Formula: C22H19N4NaO8S2
Molecular Weight: 554.53
Percent Composition: C 47.65%, H 3.45%, N 10.10%, Na 4.15%, O 23.08%, S 11.56%
Properties: Colorless needles from ethanol/water, mp 175° (dec). LD50 in mice (mg/kg): >4000 i.p.; >15000 orally (Bryskier).
Melting point: mp 175° (dec)
Toxicity data: LD50 in mice (mg/kg): >4000 i.p.; >15000 orally (Bryskier)
Derivative Type: d-Form sodium salt
Properties: [a]D23 +16.5° (c = 1.08 in water). uv max (water): 263 nm (e 14600).
Optical Rotation: [a]D23 +16.5° (c = 1.08 in water)
Absorption maximum: uv max (water): 263 nm (e 14600)
Derivative Type: l-Form sodium salt
Properties: [a]D23 -16.8° (c = 1.01 in water).
Optical Rotation: [a]D23 -16.8° (c = 1.01 in water)
Therap-Cat: Antibacterial.
Keywords: Antibacterial (Antibiotics); ?Lactams; Cephalosporins.