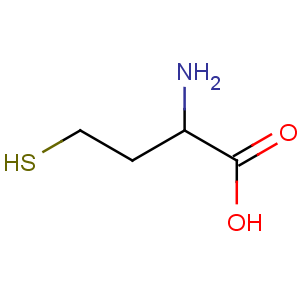
Title: Homocysteine
CAS Registry Number: 6027-13-0
CAS Name: 2-Amino-4-mercaptobutyric acid
Molecular Formula: C4H9NO2S
Molecular Weight: 135.18
Percent Composition: C 35.54%, H 6.71%, N 10.36%, O 23.67%, S 23.72%
Line Formula: HSCH2CH2CH(NH2)COOH
Literature References: A sulfur containing amino acid, produced by the demethylation of methionine and an intermediate in the biosynthesis of cysteine from methionine. Originally obtained from the liver of mammals. Has also been obtained from cystathionine: Binkley,
Methods Enzymol. 2, 314 (1955). D-Homocysteine may be prepd from
S-benzyl-D-homocysteine, while L-homocysteine is best obtained from L-homocystine: du Vigneaud, Brown,
Biochem. Prep. 5, 93 (1957),
see also Patterson, du Vigneaud,
J. Biol. Chem. 111, 393 (1935); Riegel, du Vigneaud,
ibid. 112, 149 (1935). Origin and biochemical conversions in review by Shapiro, Schlenk,
Adv. Enzymol. 22, 264-268 (1960). Use of L-form sodium salt as flavor enhancer similar to monosodium glutamate: Kaneko
et al., US 3259505 (1966 to Ajinomoto Kabushiki Kaisha).
Derivative Type: DL-Form
Properties: Platelets from dil ethanol, mp 232-233°. pK1 2.22; pK2 8.87; pK3 10.86.
Melting point: mp 232-233°
pKa: pK1 2.22; pK2 8.87; pK3 10.86
Derivative Type: Thiolactone hydrochloride
Molecular Formula: C4H8ClNOS
Molecular Weight: 153.63
Percent Composition: C 31.27%, H 5.25%, Cl 23.08%, N 9.12%, O 10.41%, S 20.87%
Properties: Crystals from abs ethanol. L-form: [a]D26 +21.5° (c = 1); D-form: [a]D26 -21.5° (c = 1).
Optical Rotation: [a]D26 +21.5° (c = 1); [a]D26 -21.5° (c = 1)