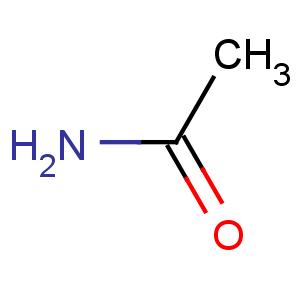
Title: Acetamide
CAS Registry Number: 60-35-5
Synonyms: Acetic acid amide
Molecular Formula: C2H5NO
Molecular Weight: 59.07
Percent Composition: C 40.67%, H 8.53%, N 23.71%, O 27.09%
Line Formula: CH3CONH2
Literature References: Prepd by fractional distillation of ammonium acetate: Coleman, Alvarado,
Org. Synth. coll. vol. I, 3 (2nd ed., 1941); Gattermann-Wieland,
Praxis des Organischen Chemikers (40th ed., 1961) p 118; Vogel,
Practical Organic Chemistry (3rd ed., 1959) p 401. Prepn from methyl acetate, W. P. Munro
et al., US 2106697 (1936 to Calco Chem.); from ethyl acetate, Vogel,
op. cit., p 403. Studies of acetamide as an ionizing solvent: Jauder, Winkler,
J. Inorg. Nucl. Chem. 9, 24, 32, 39 (1959). Toxicological study: Weisburger
et al., Toxicol. Appl. Pharmacol. 14, 163 (1969).
Properties: Deliquescent hexagonal crystals. Odorless when pure, but frequently has a mousy odor. d420 1.159. mp 81°. bp760 222°; bp100 158°; bp40 136°; bp20 120°; bp10 105°; bp5 92°.
nD78 1.4274. Neutral reaction. pKb (25°): 14.51. One gram dissolves in 0.5 ml water, 2 ml alcohol, 6 ml pyridine. Sol in chloroform, glycerol, hot benzene.
Melting point: mp 81°
Boiling point: bp760 222°; bp100 158°; bp40 136°; bp20 120°; bp10 105°; bp5 92°
pKa: pKb (25°): 14.51
Index of refraction: nD78 1.4274
Density: d420 1.159
Use: Solvent; molten acetamide is an excellent solvent for many organic and inorganic compounds. Solubilizer; renders sparingly soluble substances more soluble in water by mere addition or by fusion. Plasticizer; stabilizer. Manuf methylamine, denaturing alcohol. In organic syntheses.