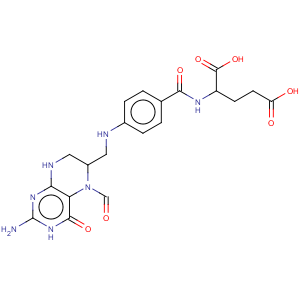
Title: Folinic Acid
CAS Registry Number: 58-05-9
CAS Name: N-[4-[[(2-Amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl]amino]benzoyl]-L-glutamic acid
Synonyms: N-[
p-[[(2-amino-5-formyl-5,6,7,8-tetrahydro-4-hydroxy-6-pteridinyl)methyl]amino]benzoyl]glutamic acid; 5-formyl-5,6,7,8-tetrahydropteroyl-L-glutamic acid; 5-formyl-5,6,7,8-tetrahydrofolic acid; CF; citrovorum factor; leucovorin
Molecular Formula: C20H23N7O7
Molecular Weight: 473.44
Percent Composition: C 50.74%, H 4.90%, N 20.71%, O 23.66%
Literature References: Intermediate product of the metabolism of folic acid; the active form into which that acid is converted in the body, ascorbic acid being a necessary factor in the conversion process. First reported as the
Leuconostoc citrovorum 8081 growth factor: H. E. Sauberlich, C. A. Baumann,
J. Biol. Chem. 176, 165 (1948). Isoln from houseflies: S. Miller, A. S. Perry,
Life Sci. 4, 1573 (1965). Prepn: J. A. Brockman, Jr.,
et al., J. Am. Chem. Soc. 72, 4325 (1950); E. Khalifa
et al., Helv. Chim. Acta 63, 2554 (1980). Isomers: D. B. Cosulich
et al., J. Am. Chem. Soc. 74, 4215 (1952). Structure: May
et al., ibid. 73, 3067 (1951); Pohland
et al., ibid. 3247. Manuf: Shive,
US 2741608 (1956 to Res. Corp.). Stereoselective synthesis: J. Owens,
et al., J. Chem. Soc. Perkin Trans. 1 7, 871 (1993). Used as an antidote to folic acid antagonists such as methotrexate,
q.v., which block the conversion of folic acid into folinic acid. Review of clinical combination therapy with methotrexate: J. R. Bertino
et al., Ann. N.Y. Acad. Sci. 186, 486-495 (1971). Pharmacokinetics: P. F. Nixon,
Clin. Exp. Pharmacol. Physiol. Suppl. 5, 35 (1979). Comprehensive description: L. O. Pont
et al., Anal. Profiles Drug Subs. 8, 315-350 (1979). Review of clinical synergy with fluorouracil in cancer: R. J. DeLap,
Yale J. Biol. Med. 61, 23-34 (1988).
Properties: Crystals, dec 240-250°. uv max (0.1
N NaOH): 282 nm (% T = 27.0 for 10 mg/l). [a]D20 +14.26° (c = 3.42 as anhydr Ca salt). pKa (3 groups): 3.1, 4.8, and 10.4. Sparingly sol in water. pH of satd aq soln 2.8-3.0 at which pH partial decompn takes place. More stable at neutral or mildly alkaline pH.
pKa: pKa (3 groups): 3.1, 4.8, and 10.4
Optical Rotation: [a]D20 +14.26° (c = 3.42 as anhydr Ca salt)
Absorption maximum: uv max (0.1
N NaOH): 282 nm (% T = 27.0 for 10 mg/l)
Derivative Type: Calcium salt pentahydrate
CAS Registry Number: 6035-45-6
Synonyms: Calcium folinate
Manufacturers' Codes: NSC-3590
Trademarks: Folaren (Ist. Chim. Inter.); Foliben (Firma); Lederfolat (Lederle); Lederfolin (Lederle); Leucovorin (Lederle); Leucosar (Adria); Rescufolin (Nordic); Rescuvolin (Medac); Tonofolin (Zyma); Wellcovorin (Burroughs Wellcome)
Molecular Formula: C20H21CaN7O7.5H2O
Molecular Weight: 601.58
Percent Composition: C 39.93%, H 5.19%, Ca 6.66%, N 16.30%, O 31.91%
Properties: Off-white to light beige amorphous, odorless powder, freely sol in water. Practically insol in alc. [a]D21 +14.9° (c = 1 in water).
Optical Rotation: [a]D21 +14.9° (c = 1 in water)
Derivative Type: l-Form calcium salt
CAS Registry Number: 80433-71-2
Synonyms: Calcium (6
S)-folinate; calcium levofolinate; levoleucovorin calcium
Trademarks: Elvorine (Lederle)
Properties: [a]D20 -15.1° (c = 1.82).
Optical Rotation: [a]D20 -15.1° (c = 1.82)
Therap-Cat: Antidote to folic acid antagonists; antianemic (folate deficiency).
Keywords: Antidote (Folic Acid Antagonists); Antineoplastic Adjunct; Folic Acid Replenisher.