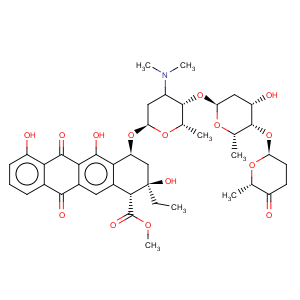
Title: Aclacinomycins
Literature References: Antitumor antibiotic complex of the anthracycline group, produced by
Streptomyces galilaeus. Thirteen yellow and seven red-colored components have been identified. Isoln of the major components, aclacinomycins A and B: H. Umezawa
et al., DE 3532568;
eidem, US 3988315 (1974, 1976 both to Microbiochem. Res. Found.).
See also: T. Oki
et al., J. Antibiot. 28, 830 (1975). Structure, taxonomy, production, properties:
eidem, ibid. 32, 791, 801 (1979). The aglycone portion of aclacinomycin A is known as
aklavinone. Isoln of aklavinone: J. J. Gordon
et al., Tetrahedron Lett. 8, 28 (1960). Synthesis of racemic aklavinone: B. A. Pearlman
et al., J. Am. Chem. Soc. 103, 4248 (1981); P. A. Confalone, G. Pizzolato,
ibid. 4251; R. K. Boeckman, F. W. Sum,
ibid. 104, 4604 (1982). Synthesis of optically active aklavinone: A. S. Kende, J. P. Rizzi,
ibid. 103, 4247 (1981); J. M. McNamara, Y. Kishi,
ibid. 104, 7371 (1982).
In vitro metabolism of aclacinomycins: T. Komiyama
et al., Gann 70, 395 (1979),
C.A. 92, 15143x (1980). HPLC determn of aclacinomycin A and its metabolites: T. Ogasawara
et al., J. Antibiot. 34, 47, 52 (1981). Pharmacokinetics: M. J. Egorin
et al., Cancer Chemother. Pharmacol. 8, 41 (1982). Series of articles on absorption, excretion, distribution and general pharmacology:
Jpn. J. Antibiot. 33, 169-213 (1980),
C.A. 93, 19316, 125326, 125649, 142986 (1980). Immunological study: M. Ishizuka
et al., J. Antibiot. 34, 331 (1981). Clinical studies: R. Maral,
Drugs Exp. Clin. Res. 9, 375 (1983); R. P. Warrell, Jr., S. J. Kempin,
Am. J. Clin. Oncol. 6, 81 (1983); A. Y. Bedikian
et al., ibid. 187 (1983). Review of pharmacology of aclacinomycin A: T. Oki,
Anthracyclines [Proc. Workshop], S. T. Crooke, S. D. Reich, Eds. (Academic Press, New York, 1980) pp 323-342,
C.A. 93, 160778h (1980).
Derivative Type: Aclacinomycin A
CAS Registry Number: 57576-44-0
CAS Name: [1
R-(1a,2b,4b)]-2-Ethyl-1,2,3,4,6,11-hexahydro-2,5,7-trihydroxy-6,11-dioxo-4-[[2,3,6-trideoxy-4-
O-[2,6-dideoxy-4-
O-[(2
R-trans)-tetrahydro-6-methyl-5-oxo-2
H-pyran-2-yl]-a-L-
lyxo-hexopyranosyl]-3-(dimethylamino)-a-L-
lyxo-hexopyranosyl]oxy]-1-naphthacenecarboxylic acid methyl ester
Synonyms: aclarubicin; antibiotic MA 144A1
Manufacturers' Codes: NSC-208734
Trademarks: Jaclacin (Medac)
Molecular Formula: C42H53NO15
Molecular Weight: 811.87
Percent Composition: C 62.13%, H 6.58%, N 1.73%, O 29.56%
Properties: Yellow microcryst powder from chloroform/hexane, mp 151-153° (dec). [a]D24 -11.5° (c = 1 in methylene chloride). uv max (methanol): 229.5, 259, 289.5, 431 nm (E1%1cm 550, 326, 135, 161); (0.1
N HCl) 229.5, 258.5, 290, 431 nm (E1%1cm 571, 338, 130, 161); (0.1
N NaOH) 239, 287, 523 nm (E1%1cm 450, 113, 127). Sol in CHCl3, ethyl acetate. Insol in ethyl ether,
n-hexane, petr ether. Addn of alkali to aq solns gives an intense reddish-purple color; in conc HCl the soln is yellow. LD50 in mice (mg/kg): 22.6 i.p., 33.7 i.v. (Oki).
Melting point: mp 151-153° (dec)
Optical Rotation: [a]D24 -11.5° (c = 1 in methylene chloride)
Absorption maximum: uv max (methanol): 229.5, 259, 289.5, 431 nm (E1%1cm 550, 326, 135, 161); (0.1
N HCl) 229.5, 258.5, 290, 431 nm (E1%1cm 571, 338, 130, 161); (0.1
N NaOH) 239, 287, 523 nm (E1%1cm 450, 113, 127)
Toxicity data: LD50 in mice (mg/kg): 22.6 i.p., 33.7 i.v. (Oki)
Derivative Type: Aclacinomycin A hydrochloride
Synonyms: Aclarubicin hydrochloride
Trademarks: Aclacin (Lundbeck); Aclacinon (Yamanouchi); Aclaplastin (Medac)
Molecular Formula: C42H53NO15.HCl
Molecular Weight: 848.33
Percent Composition: C 59.46%, H 6.42%, N 1.65%, O 28.29%, Cl 4.18%
Derivative Type: Aclacinomycin B
CAS Registry Number: 57596-79-9
CAS Name: [1
R-(1a,2b,4b)]-4-[[[2¢¢¢,3¢¢-Anhydro]-
O-3,6-dideoxy-a-L-
erythro-hexopyranos-4-ulos-1-yl-(1?4)-
O-2,6-dideoxy-a-L-
lyxo-hexopyranosyl-(1?4)-2,3,6-trideoxy-3-(dimethylamino)-a-L-
lyxo-hexopyranosyl]oxy]-2-ethyl-1,2,3,4,6,11-hexahydro-2,5,7-trihydroxy-6,11-dioxo-1-naphthacenecarboxylic acid methyl ester
Synonyms: antibiotic MA 144B1
Molecular Formula: C42H51NO15
Molecular Weight: 809.85
Percent Composition: C 62.29%, H 6.35%, N 1.73%, O 29.63%
Properties: Yellow microcryst powder from chloroform/hexane, mp 163-167° (dec). [a]D24 +3° (c = 1 in methylene chloride). Other physical properties similar to aclacinomycin A. LD50 in mice (mg/kg): 13.7 i.p., 16.4 i.v. (Oki).
Melting point: mp 163-167° (dec)
Optical Rotation: [a]D24 +3° (c = 1 in methylene chloride)
Toxicity data: LD50 in mice (mg/kg): 13.7 i.p., 16.4 i.v. (Oki)
Therap-Cat: Antineoplastic.
Keywords: Antineoplastic; Antibiotics and Analogs; Anthracyclines.