References of Ergotaman-3',6',18-trione,12'-hydroxy-2',5'-bis(1-methylethyl)-, (5'a)-
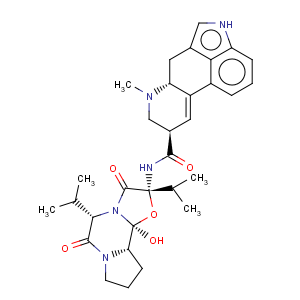
Title: Ergocornine
CAS Registry Number: 564-36-3
CAS Name: (5¢a)-12¢-Hydroxy-2¢,5¢-bis(1-methylethyl)ergotaman-3¢,6¢,18-trione
Molecular Formula: C31H39N5O5
Molecular Weight: 561.67
Percent Composition: C 66.29%, H 7.00%, N 12.47%, O 14.24%
Literature References: Natural ergot alkaloid derived from lysergic acid; component of ergotoxine,
q.v. Isoln from ergot: Stoll, Hofmann,
Helv. Chim. Acta 26, 1570 (1943). Structure: Stoll
et al., ibid. 34, 1544 (1951). Separation and purification: Stoll, Hofmann,
US 2447214 (1948 to Sandoz). Synthesis: Stadler
et al., Helv. Chim. Acta 52, 1549 (1969). Determn in ergot by capillary electrophoresis: K. Frach, G. Blaschke,
J. Chromatogr. A 808, 247 (1998).
Properties: Solvated, polyhedra from methanol, dec 181° (contains 1 mole methanol). [a]D20 -110° (pyridine); -175° (chloroform). uv max (methanol): 311 nm (log e 3.91). Soluble in acetone, chloroform, ethyl acetate; slightly sol in ethyl and methyl alcohol. Nearly insol in water.
Optical Rotation: [a]D20 -110° (pyridine); -175° (chloroform)
Absorption maximum: uv max (methanol): 311 nm (log e 3.91)
Derivative Type: 8a-Epimer
CAS Registry Number: 564-37-4
Synonyms: Ergocorninine
Properties: Prisms from alc, dec 228°. [a]D20 +409° (chloroform). uv max (methanol): 240.5, 312.5 (log e 4.31, 3.92). Soluble in 15 parts boiling ethanol, 25 parts boiling methanol, 30 parts boiling benzene, 30 parts boiling ethyl acetate; freely sol in acetone, chloroform. Practically insol in water.
Optical Rotation: [a]D20 +409° (chloroform)
Absorption maximum: uv max (methanol): 240.5, 312.5 (log e 4.31, 3.92)