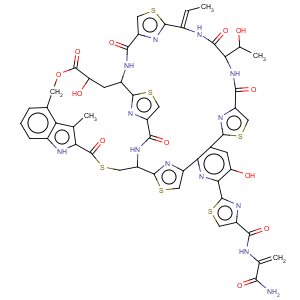
Title: Nosiheptide
CAS Registry Number: 56377-79-8
Synonyms: Multhiomycin
Manufacturers' Codes: RP-9671
Trademarks: Primofax (Rh>e-Poulenc)
Molecular Formula: C51H43N13O12S6
Molecular Weight: 1222.36
Percent Composition: C 50.11%, H 3.55%, N 14.90%, O 15.71%, S 15.74%
Literature References: Polythiazole antibiotic produced by
Streptomyces actuosus. Isoln and characterization: S. Pinnert
et al., FR 1392453;
eidem, US 3155581 (1961, 1964 both to Rh?ne-Poulenc); F. Benazet
et al., Experientia 36, 414 (1980). NMR determination of mol wt and elemental formula: H. Depaire
et al., Tetrahedron Lett. 1977, 1397, 1401. Structure and configuration: T. Prange
et al., Nature 265, 189 (1977); C. Pascard
et al., J. Am. Chem. Soc. 99, 6418 (1977). Biosynthetic study: D. P. Houck
et al., ibid. 109, 1250 (1987). Identity with multhiomycin: T. Endo, H. Yonehara,
J. Antibiot. 31, 623 (1978). Mode of action: E. Cundliffe, J. Thompson,
J. Gen. Microbiol. 126, 185 (1981).
Review: F. Benazet
et al., Experientia 36, 414-416 (1980).
Properties: Yellow needles, mp 310-320° (dec). [a]D20 +38° (c = 1 in pyridine). uv max (water/DMF): 242, 322 nm (E1%1cm 525, 229). Sol in chloroform, dioxane, pyridine, DMF, DMSO; slightly sol in methanol, ethanol, ethyl acetate, benzene. Insol in water and petr ether.
Melting point: mp 310-320° (dec)
Optical Rotation: [a]D20 +38° (c = 1 in pyridine)
Absorption maximum: uv max (water/DMF): 242, 322 nm (E1%1cm 525, 229)
Therap-Cat-Vet: Antibacterial; growth promotant.