Title: Glycerol
CAS Registry Number: 56-81-5
CAS Name: 1,2,3-Propanetriol
Synonyms: glycerin; glycerine; trihydroxypropane; incorporation factor; IFP
Trademarks: Bulbold (Sandoz-Wander); Cristal (Vifor); Glyceol (Chugai); Ophthalgan (Wyeth-Ayerst)
Molecular Formula: C3H8O3
Molecular Weight: 92.09
Percent Composition: C 39.13%, H 8.76%, O 52.12%
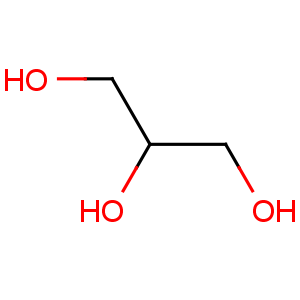
Line Formula: CH2OHCHOHCH2OH
Literature References: Obtained from oils and fats as byproduct in the manuf of soaps and fatty acids. During World War I supplementary quantities were produced by the "Protol" fermentation process from sugar, a process based upon the fixation of acetaldehyde by sodium sulfite. Just prior to World War II the synthesis of glycerol from propylene was announced. Production from sugars by fermentation: Onishi,
US 3012945 (1961 to Noda). Identity with incorporation factor: Kuehl
et al., J. Am. Chem. Soc. 82, 2079 (1960). In nucleic acid the incorporation factor may exist as a bound form of glycerol. Acute toxicity: W. Bartsch
et al., Arzneim.-Forsch. 26, 1581 (1976). Reviews and bibliographies: J. W. Lawrie,
Glycerol and the Glycols (New York, 1928); G. Leffingwell, M. Lesser,
Glycerin (Brooklyn, 1945); C. S. Miner, N. N. Dalton,
Glycerol (New York, 1953); C. Lüttgen,
Glyzerin und glyzerin?hnliche Stoffe (Heidelberg, 2nd ed., 1955); J. C. Kern in
Kirk-Othmer Encyclopedia of Chemical Technology vol. 11 (Wiley-Interscience, New York, 3rd ed., 1980) pp 921-932.
Properties: Syrupy liquid. Sweet warm taste. About 0.6 times as sweet as cane sugar. Hygroscopic; also absorbs H2S, HCN, SO2.
Contact with strong oxidizing agents such as chromium trioxide, potassium chlorate, or potassium permanganate may produce an explosion. Neutral to litmus. Solidifies after prolonged cooling at 0° forming shiny orthorhombic crystals, mp 17.8°. bp760 290.0° (dec); bp400 263.0°; bp200 240.0°; bp100 220.1°; bp60 208.0°; bp20 182.2°; bp10 167.2°; bp5 153.8°; bp1.0 125.5°.
nD15 1.4758;
nD20 1.4746;
nD25 1.4730. d1515 1.26557; d2020 1.26362; d2525 1.26201. Flash point, open cup: 350°F (176°C). Specific gravities of 95% aq soln w/w (U.S.P. grade): d1515 1.25270; d2020 1.25075; d2525 1.24910; 90% aq soln w/w: d1515 1.23950; d2020 1.23755; d2525 1.23585; 80% d1515 1.213; 70% d1515 1.185; 60% d1515 1.157; 50% d1515 1.129; 20% d1515 1.049; 5% d1515 1.0122. Viscosity (cP at 20°): 5% soln 1.143; 10% 1.311; 25% 2.095; 50% 6.050; 60% 10.96; 70% 22.94; 83% 111. Freezing points of aq solns w/w: 10% -1.6°; 30% -9.5°; 50% -23.0°; 66.7% -46.5°; 80% -20.3°; 90% -1.6°. Miscible with water, alcohol. One part dissolves in 11 parts ethyl acetate, in about 500 parts ethyl ether. Insol in benzene, chloroform, carbon tetrachloride, carbon disulfide, petr ether, oils. LD50 in rats (ml/kg): >20 orally; 4.4 i.v. (Bartsch).
Melting point: mp 17.8°
Boiling point: bp760 290.0° (dec); bp400 263.0°; bp200 240.0°; bp100 220.1°; bp60 208.0°; bp20 182.2°; bp10 167.2°; bp5 153.8°; bp1.0 125.5°
Flash point: Flash point, open cup: 350°F (176°C)
Index of refraction: nD15 1.4758;
nD20 1.4746;
nD25 1.4730
Density: d1515 1.26557; d2020 1.26362; d2525 1.26201; 95% aq soln w/w (U.S.P. grade): d1515 1.25270; d2020 1.25075; d2525 1.24910; 90% aq soln w/w: d1515 1.23950; d2020 1.23755; d2525 1.23585; 80% d1515 1.213; 70% d1515 1.185; 60% d1515 1.157; 50% d1515 1.129; 20% d1515 1.049; 5% d1515 1.0122
Toxicity data: LD50 in rats (ml/kg): >20 orally; 4.4 i.v. (Bartsch)
CAUTION: Potential symptoms of overexposure to mist are irritation of eyes, skin, respiratory system; headache, nausea, vomiting; kidney injury.
See NIOSH Pocket Guide to Chemical Hazards (DHHS/NIOSH 97-140, 1997) p 152.
Use: As solvent, humectant, plasticizer, emollient, sweetener, in the manuf of nitroglycerol (dynamite), cosmetics, liq soaps, liqueurs, confectioneries, blacking, printing and copying inks, lubricants, elastic glues, lead oxide cements; to keep fabrics pliable; to preserve printing on cotton; for printing rollers, hectographs; to keep frost from windshields; as antifreeze in automobiles, gas meters and hydraulic jacks, in shock absorber fluids. In fermentation nutrients in the production of antibiotics. Pharmaceutic aid (humectant; solvent, vehicle). Leffingwell and Lesser (
op. cit.) give 1583 different uses.
Therap-Cat: Diagnostic aid (ophthalmic).
Therap-Cat-Vet: Emollient, demulcent. As a source of glucose in bovine ketosis.
Keywords: Diagnostic Aid.