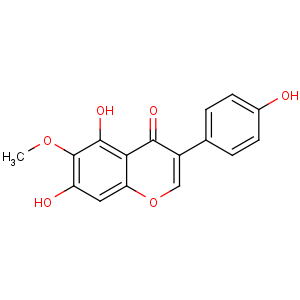
Title: Tectorigenin
CAS Registry Number: 548-77-6
CAS Name: 5,7-Dihydroxy-3-(4-hydroxyphenyl)-6-methoxy-4
H-1-benzopyran-4-one
Synonyms: 4¢,5,7-trihydroxy-6-methoxyisoflavone
Molecular Formula: C16H12O6
Molecular Weight: 300.26
Percent Composition: C 64.00%, H 4.03%, O 31.97%
Literature References: Phytoalexin aglycon isolated from rhizomes of
Iris tectorum Maxim., Iridaceae, the extract of which is used as a traditional Chinese medicine. Isoln of the glycoside and aglycon: S. Shibata,
J. Pharm. Soc. Jpn. 37, 380 (1927),
C.A. 21, 30508 (1927); from
Iris germanica Linnaeus: A. Kawase
et al., Agric. Biol. Chem. 37, 145 (1973). Structure: Asahina
et al., J. Pharm. Soc. Jpn. 48, 1087 (1928),
C.A. 23, 27181 (1929); Shriner
et al., J. Am. Chem. Soc. 61, 2322 (1939). Synthesis: Farkas, Várady,
Ber. 93, 1269 (1960); W. Baker
et al., J. Chem. Soc. C 1970, 1219. Inhibition of cyclooxygenase-2 induction: K. Ohuchi
et al., Recent Adv. Nat. Prod. Res., Proc. 3rd Int. Symp. 1999, 12-24. Antioxidant effects: K.-T. Lee
et al., Arch. Pharmacal Res. 23, 461 (2000).
Properties: Pale yellow needles from ethanol, mp 225-226°. uv max (ethanol): 267-269, 338 nm (log e 4.41, 3.40).
Melting point: mp 225-226°
Absorption maximum: uv max (ethanol): 267-269, 338 nm (log e 4.41, 3.40)
Derivative Type: Tectoridin
CAS Registry Number: 611-40-5
Synonyms: Tectorigenin-7-glucoside; shekanin
Molecular Formula: C22H22O11
Molecular Weight: 462.40
Percent Composition: C 57.14%, H 4.80%, O 38.06%
Literature References: Identity with shekanin and structure: Mannich
et al., Arch. Pharm. 275, 317 (1937). Synthesis: Várady,
Acta Chim. Acad. Sci. Hung. 48, 181 (1966).
Properties: Colorless needles from ethanol, mp 256-258°. uv max (ethanol): 267-269, 336 nm (log e 4.62, 3.65). [a]D20 -29.4° (pyridine). Sol in pyridine, alcohol, water. Practically insol in organic solvents.
Melting point: mp 256-258°
Optical Rotation: [a]D20 -29.4° (pyridine)
Absorption maximum: uv max (ethanol): 267-269, 336 nm (log e 4.62, 3.65)