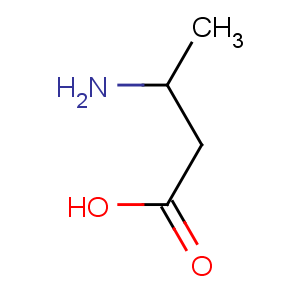
Title: b-Aminobutyric Acid
CAS Registry Number: 541-48-0
CAS Name: 3-Aminobutanoic acid
Synonyms: b-amino-
n-butyric acid
Molecular Formula: C4H9NO2
Molecular Weight: 103.12
Percent Composition: C 46.59%, H 8.80%, N 13.58%, O 31.03%
Line Formula: CH3CH(NH2)CH2COOH
Literature References: Prepd from pyrotartaric acid diamide: Weidel, Roithmer,
Monatsh. Chem. 17, 185 (1896); from b-chlorobutyric acid ethyl ester and alcoholic NH3: Balbiano,
Ber. 13, 312 (1880); from crotonic acid and concd NH3: Engel,
Bull. Soc. Chim. [2]
50, 102 (1888); Curtius, Gumlich,
J. Prakt. Chem. [2]
70, 204 (1904); Stadnikow,
Chem. Zentralbl. 1909, II, 1988;
see Fischer, Roeder,
Ber. 34, 3755 (1901) footnote; Stoermer, Robert,
Ber. 55, 1038 (1922). Prepn of HCl salt from b-aminobutyronitrile: Bruylants,
Bull. Soc. Chim. Belg. 32, 259 (1923);
Chem. Zentralbl. 1924, I, 1668. By reducing acetoacetic ester phenylhydrazone and saponifying the resulting ester: Fischer, Groh,
Ann. 383, 338 (1911). The D(-)-form has been obtained by hydrolysis of its ester: Fischer, Scheibler,
ibid. 346.
Derivative Type: DL-Form
Properties: Crystals from alcohol, mp 193-194°. Practically tasteless. Sol in water. One liter of water dissolves 1250 g. Insol in cold absolute alcohol and ether.
Melting point: mp 193-194°
Derivative Type: Methyl ester
Molecular Formula: C5H11NO2
Molecular Weight: 117.15
Percent Composition: C 51.26%, H 9.46%, N 11.96%, O 27.31%
Properties: Odoriferous liquid; d20 0.993; bp13 54-55°. Sol in water, alcohol, ether, petr ether.
Boiling point: bp13 54-55°
Density: d20 0.993
Derivative Type: D-Form
Properties: Prisms from methanol. Dec near 220° without melting. [a]D20 -35.20° (p = 10).
Optical Rotation: [a]D20 -35.20° (p = 10)