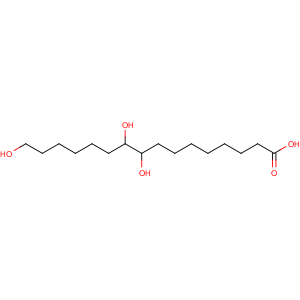
Title: Aleuritic Acid
CAS Registry Number: 533-87-9
CAS Name: (9
R,10
S)-
rel-9,10,16-Trihydroxyhexadecanoic acid
Synonyms: 9,10,16-trihydroxypalmitic acid; 8,9,15-trihydroxypentadecane-1-carboxylic acid
Molecular Formula: C16H32O5
Molecular Weight: 304.42
Percent Composition: C 63.13%, H 10.60%, O 26.28%
Literature References: One of the constituent acids of shellac. Obtained in 43% yield from dewaxed shellac: Schaeffer, Gardner,
Ind. Eng. Chem. 30, 333 (1938); Gidvani,
J. Chem. Soc. 1944, 306; Sengupta, Bose,
J. Sci. Ind. Res. 11B, 458 (1952). The acid obtained from shellac is optically inactive, although it contains two asymmetric carbon atoms. It has been shown to be the DL
-erythro or
dl-cis form, and is the only form described here. Synthesis of diastereoisomers: Mitter
et al., Sci. Cult. 8, 273 (1942); Hunsdiecker,
Ber. 76, 142 (1943);
77, 185 (1944); Baudart,
Compt. Rend. 221, 205 (1945).
Properties: Crystals from dilute ethanol, mp 100-101°. Sol in methanol. Forms a crystalline sodium salt.
Melting point: mp 100-101°
Derivative Type: Methyl ester
Molecular Formula: C17H34O5
Molecular Weight: 318.45
Percent Composition: C 64.12%, H 10.76%, O 25.12%
Properties: Fine feathery needles, mp 72-73°; bp0.1 235°. Sol in methanol, ethanol, chloroform, acetone. Less sol in benzene. Insol in petr ether.
Melting point: mp 72-73°
Boiling point: bp0.1 235°
Derivative Type: Ethyl ester
CAS Registry Number: 6003-09-4
Molecular Formula: C18H36O5
Molecular Weight: 332.48
Percent Composition: C 65.02%, H 10.91%, O 24.06%
Properties: Needles from dil ethanol, mp 59°.
Melting point: mp 59°
Derivative Type: Hydrazide
CAS Registry Number: 6003-10-7
Molecular Formula: C16H34N2O4
Molecular Weight: 318.45
Percent Composition: C 60.35%, H 10.76%, N 8.80%, O 20.10%
Properties: Crystals from abs ethanol, mp 139-140°.
Melting point: mp 139-140°