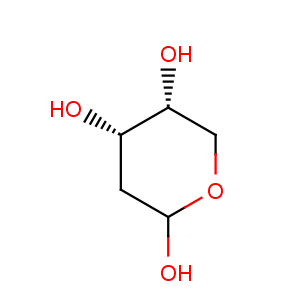
Title: D-2-Deoxyribose
CAS Registry Number: 533-67-5
CAS Name: 2-Deoxy-D-
erythro-pentose
Synonyms: desoxyribose; D-2-deoxyarabinose; D-2-ribodesose; D-
erythro-2-deoxypentose; thyminose
Molecular Formula: C5H10O4
Molecular Weight: 134.13
Percent Composition: C 44.77%, H 7.51%, O 47.71%
Literature References: Isoln from deoxyribonucleic acid by acidic hydrolysis of purine deoxyribonucleosides which have been isolated by ion-exchange resin chromatography: Laland, Overend,
Acta Chem. Scand. 8, 192 (1954). Synthesis: Felton, Freudenberg,
J. Am. Chem. Soc. 57, 1637 (1935); Deriaz
et al., J. Chem. Soc. 1949, 1879, 2836; Hough,
Chem. Ind. (London) 1951, 406; Sowden,
Biochem. Prep. 5, 75 (1957); I. Ziderman, E. Dimant,
J. Org. Chem. 32, 1267 (1967); J. R. Hauske, H. Rapoport,
ibid. 44, 2472 (1979); T. Harada, T. Mukaiyama,
Chem. Lett. 1981, 1109.
Review: Overend, Stacey, in Chargaff-Davidson,
Nucleic Acids vol. 1, E. Chargaff, N. J. Davidson, Eds. (Academic Press, New York, 1955) pp 1-80.
Properties: Crystals from isopropanol, mp 91°. Shows mutarotation. Final [a]D22 -56.2° (H2O). Sol in water, pyridine. Slightly sol in alc.
Melting point: mp 91°
Optical Rotation: [a]D22 -56.2° (H2O)
Derivative Type: 1,3,4-Triacetate
Molecular Formula: C11H16O7
Molecular Weight: 260.24
Percent Composition: C 50.77%, H 6.20%, O 43.04%
Properties: Needles from methanol, mp 98°. [a]D23 -171.8° (c = 0.56 in chloroform): Allerton, Overend,
J. Chem. Soc. 1951, 1480.
Melting point: mp 98°
Optical Rotation: [a]D23 -171.8° (c = 0.56 in chloroform): Allerton, Overend,
J. Chem. Soc. 1951, 1480
Derivative Type: 3,4,5-Triacetate
Properties: Oily liq, bp0.001 105°. [a]D21 +3.4° (c = 4.57 in pyridine): Zinner
et al., Ber. 90, 2696 (1957).
Boiling point: bp0.001 105°
Optical Rotation: [a]D21 +3.4° (c = 4.57 in pyridine): Zinner
et al., Ber. 90, 2696 (1957)
Derivative Type: 1,3,4-Tribenzoate
Properties: Small white nodules from ethanol, mp 127°. [a]D23 -65° (c = 1.02 in chloroform) (Allerton, Overend). Probably a mixture of the two anomeric 2-deoxy-D-ribopyranose tribenzoates: Pedersen
et al., J. Am. Chem. Soc. 82, 3425 (1960).
Melting point: mp 127°
Optical Rotation: [a]D23 -65° (c = 1.02 in chloroform) (Allerton, Overend)
Derivative Type: 3,4,5-Tribenzoate
Properties: Fine needles from ethyl acetate + petr ether, mp 118-119°. [a]D18 -2.8° (c = 1.44 in pyridine) (Zinner).
Melting point: mp 118-119°
Optical Rotation: [a]D18 -2.8° (c = 1.44 in pyridine) (Zinner)