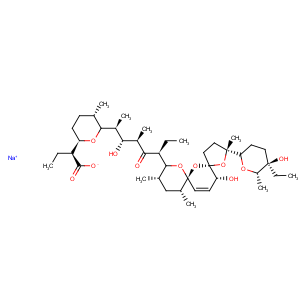
Title: Salinomycin
CAS Registry Number: 53003-10-4
Molecular Formula: C42H70O11
Molecular Weight: 751.00
Percent Composition: C 67.17%, H 9.39%, O 23.43%
Literature References: Polyether ionophoric antibiotic having a unique tricyclic spiroketal ring system and an unsaturated 6-membered ring in the molecule. Produced by a strain of
Streptomyces albus (FERM-P No. 419 and ATCC 21838). Production: Y. Miyazaki
et al., JP Kokai 72 25392 (1972 to Kaken Chem.),
C.A. 78, 41561 (1973). Structure: H. Kinashi
et al., Tetrahedron Lett. 1973, 4955. Taxonomy, production, isolation and physicochemical and biological properties: Y. Miyazaki
et al., J. Antibiot. 27, 814 (1974). Use as a coccidiostat: Y. Tanaka
et al., DE 2253031;
eidem, US 3857948 (1973, 1974 to Kaken Chem.). Anticoccidial efficacy: T. T. Migaki
et al., Poult. Sci. 58, 1192 (1979). Total synthesis: Y. Kishi
et al., Front. Chem., Plenary Keynote Lect. IUPAC Congr., 28th 1981, K. J. Laidler, Ed. (Pergamon, Oxford, 1982) pp 287-304; R. C. D. Brown, P. J. Kocienski,
Synlett 1994, 415, 417. HPLC determn in animal feeds: M. R. LaPointe, H. Cohen,
J. Assoc. Off. Anal. Chem. 71, 480 (1988).
Properties: mp 112.5-113.5°. pKa¢ 6.4 (DMF). [a]D25 -63° (c = 1 in ethanol). uv max (ethanol-water, 2:1): 284 nm (e 126). LD50 in mice (mg/kg): 18 i.p.; 50 orally (Miyazaki).
Melting point: mp 112.5-113.5°
pKa: pKa¢ 6.4 (DMF)
Optical Rotation: [a]D25 -63° (c = 1 in ethanol)
Absorption maximum: uv max (ethanol-water, 2:1): 284 nm (e 126)
Toxicity data: LD50 in mice (mg/kg): 18 i.p.; 50 orally (Miyazaki)
Derivative Type: Sodium salt
CAS Registry Number: 55721-31-8
Trademarks: Bio-Cox (Alpharma); Sacox (Intervet); Salocin (Intervet)
Molecular Formula: C42H69NaO11
Molecular Weight: 772.98
Percent Composition: C 65.26%, H 9.00%, Na 2.97%, O 22.77%
Properties: mp 140-142°. [a]D25 -37° (c = 1 in ethanol).
Melting point: mp 140-142°
Optical Rotation: [a]D25 -37° (c = 1 in ethanol)
Therap-Cat-Vet: Anticoccidial agent.