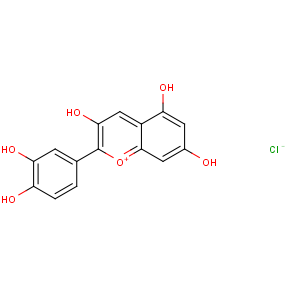
Title: Cyanidin Chloride
CAS Registry Number: 528-58-5
CAS Name: 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopyrylium chloride
Synonyms: 3,3¢,4¢,5,7-pentahydroxyflavylium chloride; 3,3¢,4¢,5,7-pentahydroxy-2-phenylbenzopyrylium chloride
Molecular Formula: C15H11ClO6
Molecular Weight: 322.70
Percent Composition: C 55.83%, H 3.44%, Cl 10.99%, O 29.75%
Literature References: Prepd by acid hydrolysis of cyanin chloride: Willst?tter, Everest,
Ann. 401, 189 (1913). Isoln from bananas: Simmonds,
Nature 173, 402 (1954). Prepn by reduction of quercetin: Bauer
et al., Chem. Ind. (London) 1954, 433; King, White,
J. Chem. Soc. 1957, 3901. Structure: Willst?tter, Mallison,
Ann. 408, 147 (1915). Synthesis: Willst?tter
et al., Ber. 57, 1938 (1924); Robertson, Robinson,
J. Chem. Soc. 1928, 1528. Biosynthesis: Fritsch
et al., Z. Naturforsch. 26b, 581 (1971).
See also Bioflavonoids.
Properties: Metallic brownish-red needles (monohydrate) from dil alcoholic HCl. The anhydr compd does not melt below 300°. Absorption max (methanolic HCl): 535 nm. Freely sol in alcohol and in amyl alcohol giving a violet soln. Sol in sodium carbonate soln with blue color. Sparingly sol in dil HCl or H2SO4.
Absorption maximum: Absorption max (methanolic HCl): 535 nm
Derivative Type: 3-Glucoside
Synonyms: Chrysanthemin; asterin; kuromanin
Molecular Formula: C21H21ClO11
Molecular Weight: 484.84
Percent Composition: C 52.02%, H 4.37%, Cl 7.31%, O 36.30%
Properties: From winter aster
(Chrysanthemum indicum L.,
Compositae): Willst?tter, Bolton,
Ann. 412, 136 (1917); from wild strawberries
(Fragaria vesca L.,
Rosaceae): Sondheimer, Karash,
Nature 178, 648 (1956); from sweet cherries
(Prunus avium L.,
Rosaceae): Li, Wagenknecht
ibid. 182, 657 (1958). Identity with asterin: Robinson, Willst?tter,
Ber. 61, 2503 (1928). Structure and synthesis: Murakami
et al., J. Chem. Soc. 1931, 2665. Reddish-brown plates or prisms with a metallic shine from dil alcoholic HCl, dec 205° without melting. Absorption max (methanolic HCl): 525 nm. Sol in alc with strong fluorescence and in sodium carbonate with violet-blue color.
Absorption maximum: Absorption max (methanolic HCl): 525 nm
Derivative Type: 3-Rhamnoglucoside
Synonyms: Keracyanin; antirrhinin; sambucin
Trademarks: Meralop (ISF); Meralops (Dulcis)
Molecular Formula: C27H31ClO15
Molecular Weight: 630.98
Percent Composition: C 51.39%, H 4.95%, Cl 5.62%, O 38.03%
Properties: From skin of sweet cherries
(Prunus avium L,.
Rosaceae): Willst?tter, Zollinger,
Ann. 412, 164 (1917); from sour cherries
(Prunus cerasus L.,
Rosaceae): Li, Wagenknecht,
J. Am. Chem. Soc. 78, 979 (1956). Structure: Robertson, Robinson,
J. Chem. Soc. 1927, 2196. Prepn by reduction of quercetin-3-rhamnoglucoside: Bauer
et al., Chem. Ind. (London) 1954, 433. Reduces time to adjust to darkness: F. Trimarchi
et al., Min. Oftalmol. 18, 143 (1977). Mechanism of action: F. Trimarchi
et al., Ann. Ottalmol. Clin. Ocul. 105, 111 (1979). Red needles from dil HCl or dark prisms from dil methanolic HCl. Absorption max (ethanolic HCl): 532, 333, 282 nm. Sol in hot water, alcohol.
Absorption maximum: Absorption max (ethanolic HCl): 532, 333, 282 nm
Derivative Type: 3-Galactoside
Synonyms: Idaein; idein
Molecular Formula: C21H21ClO11
Molecular Weight: 484.84
Percent Composition: C 52.02%, H 4.37%, Cl 7.31%, O 36.30%
Properties: From cranberries
(Vaccinium vitis idaea L.,
Ericaceae): Willst?tter, Mallison,
Ann. 408, 15 (1915); from Winesap apple
(Pyrus malus Linn.,
Rosaceae): Duncan, Dustman,
J. Am. Chem. Soc. 58, 1511 (1936). Structure and synthesis: Grove, Robinson,
J. Chem. Soc. 1931, 2722. Red needles with bronze metallic luster from dil ethanolic HCl, dec 210-212°. Sol in water, ethanol, methanol, dil HCl. Practically insol in 7% HCl.
Derivative Type: 3,5-Diglucoside
Synonyms: Cyanin; shisonin A
Molecular Formula: C27H31ClO16
Molecular Weight: 646.98
Percent Composition: C 50.12%, H 4.83%, Cl 5.48%, O 39.57%
Properties: From cornflower (
Centaurea cyanus L.,
Compositae): Willst?tter, Everest,
loc. cit. Structure: Léon, Robinson,
J. Chem. Soc. 1932, 221. Synthesis: Robinson, Todd,
ibid. 2488. Plates with a metallic luster from dil alcoholic HCl, mp 203-204°. [a]D -258° (in 0.05% HCl). Absorption max (methanolic HCl): 522 nm.
Melting point: mp 203-204°
Optical Rotation: [a]D -258° (in 0.05% HCl)
Absorption maximum: Absorption max (methanolic HCl): 522 nm
Derivative Type: 3-Sophoroside
Synonyms: Mecocyanin
Molecular Formula: C27H31ClO16
Molecular Weight: 646.98
Percent Composition: C 50.12%, H 4.83%, Cl 5.48%, O 39.57%
Properties: From the flowers of
Papaver rhoeas L.,
Papaveraceae: Willst?tter, Weil,
Ann. 412, 231 (1917); from sour cherries: Li, Wagenknecht,
J. Am. Chem. Soc., loc. cit. Structure: Harborne,
Experientia 19, 7 (1963);
Phytochemistry 2, 85 (1963). (Alternate structure: Grove
et al., J. Chem. Soc. 1934, 1608). Dark-red crystals from dil HCl + HOAc or dark-red needles from 2% alcoholic HCl. Absorption max (methanolic HCl): 523 nm. Sol in water. Pptd by glacial acetic acid or acetone.
Absorption maximum: Absorption max (methanolic HCl): 523 nm
Therap-Cat: 3-Rhamnoglucoside in treatment of night blindness.