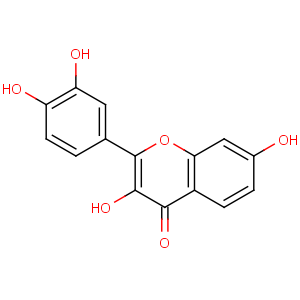
Title: Fisetin
CAS Registry Number: 528-48-3
CAS Name: 2-(3,4-Dihydroxyphenyl)-3,7-dihydroxy-4
H-1-benzopyran-4-one
Synonyms: 3,3¢,4¢,7-tetrahydroxyflavone; 5-desoxyquercetin; fisidenolon 1521; C.I. 75620; C.I. Natural Brown 1
Molecular Formula: C15H10O6
Molecular Weight: 286.24
Percent Composition: C 62.94%, H 3.52%, O 33.54%
Literature References: Flavanoid present in the bark and stems of a variety of trees. Isolation from
Rhus cotinus L.
Anacardiaceae, Venetian Sumach: Chevreul,
Lecons Chim. Appl. a la Teint. 2, 169 (1833) [
cf. J. Schmid,
Ber. 19, 1734 (1886)]; from heartwood of
Acacia spp,
Leguminosae: Roux
et al., Biochem. J. 78, 834 (1961). Structure: Seshadri,
Annu. Rev. Biochem. 20, 492 (1951). Synthesis: St. Kostanecki
et al., Ber. 37, 784 (1904); J. Allan, R. Robinson,
J. Chem. Soc. 129, 2334 (1926). Inhibition of aflatoxin cytotoxicity: A. G. Schwartz, W. R. Rate,
J. Environ. Pathol. Toxicol. 2, 1021 (1979). Mutagenicity studies: J. P. Brown
et al., Biochem. Soc. Trans. 5, 1489 (1977);
idem, P. S. Dietrich,
Mutat. Res. 66, 223 (1979). TLC determn: D. Heimler,
J. Chromatogr. 366, 407 (1986).
Properties: Yellow needles from dil alc, dec 330°. uv max (ethanol): 252, 320, 360 nm (log E1%1cm 2.62, 2.51, 2.73). Sol in alcohol, acetone, acetic acid, solns of fixed alkali hydroxides. Practically insol in water, ether, benzene, chloroform and petr ether.
Absorption maximum: uv max (ethanol): 252, 320, 360 nm (log E1%1cm 2.62, 2.51, 2.73)