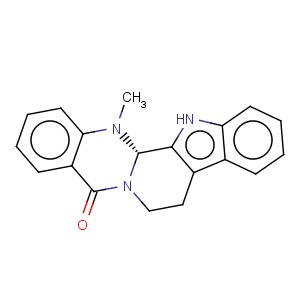
Title: Evodiamine
CAS Registry Number: 518-17-2
CAS Name: 8,13,13b,14-Tetrahydro-14-methylindolo[2¢,3¢:3,4]pyrido[2,1-
b]quinazolin-5(7
H)-one
Molecular Formula: C19H17N3O
Molecular Weight: 303.36
Percent Composition: C 75.23%, H 5.65%, N 13.85%, O 5.27%
Literature References: From
Evodia rutaecarpa Hook. & Thoms and bark of
Zanthoxylum rhetsa DC.,
Rutaceae: Y. Asahina, K. Kashiwaki,
J. Pharm. Soc. Jpn. 1915, 1293,
C.A. 10, 607 (1916); Gopinath
et al., Tetrahedron 8, 293 (1960). Structure: Y. Asahina
J. Pharm. Soc. Jpn. 1924, 1; Ohta,
J. Pharm. Soc. Jpn. 65, 15 (1945),
C.A. 45, 5697 (1951). Synthesis: Asahina, Ohta,
Ber. 61B, 319 (1928); T. Kametani
et al., J. Am. Chem. Soc. 98, 6186 (1976);
eidem, Heterocycles 4, 23 (1976). Biosynthesis: M. Yamazaki
et al., Tetrahedron Lett. 1966, 3221;
1967, 3317. Mass spec.: J. Tamas
et al., Acta Chim. Acad. Sci. Hung. 89, 85 (1976).
Properties: Yellow plates from alc, mp 278°. [a]D15 +352° (acetone); [a]D +440° (chloroform). uv max (acetonitrile): 272, 280, 291, 335 nm (log e 4.06, 4.02, 3.90, 3.30). Sol in acetone; slightly sol in alcohol, ether, chloroform. Practically insol in water, petr ether, benzene. Does not seem to form salts.
Melting point: mp 278°
Optical Rotation: [a]D15 +352° (acetone); [a]D +440° (chloroform)
Absorption maximum: uv max (acetonitrile): 272, 280, 291, 335 nm (log e 4.06, 4.02, 3.90, 3.30)