References of Pyrrolidinium,2-carboxy-4-hydroxy-1,1-dimethyl-, inner salt, (2S,4R)-
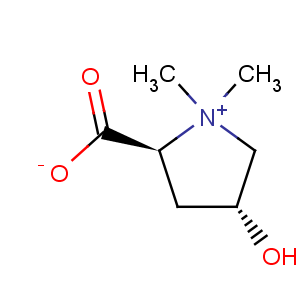
Title: Betonicine
CAS Registry Number: 515-25-3
CAS Name: (2
S-
trans)-2-Carboxy-4-hydroxy-1,1-dimethylpyrrolidinium inner salt
Synonyms: l-N,N-dimethyl-4-hydroxypyrrolidine-2-carboxylic acid betaine;
l-1-methyl-4-hydroxypyrrolidine-2-carboxylic acid methylbetaine;
l-4-hydroxystachydrine;
l-4-hydroxyproline betaine
Molecular Formula: C7H13NO3
Molecular Weight: 159.18
Percent Composition: C 52.82%, H 8.23%, N 8.80%, O 30.15%
Literature References: Occurs in
Croton gubouga S. Moore; in
Stachys officinalis (L.) Trev.
(Betonica officinalis L.
), Labiatae; in
Achillea moschata Jacq. and
A. millefolium L.,
Compositae: Goodson, Clewer,
J. Chem. Soc. 115, 923 (1919); Guggenheim,
Die biogenen Amine (S. Karger, New York, 1951) p 246; Miller, Chow,
J. Am. Chem. Soc. 76, 1353 (1954); Pailer, Kump,
Monatsh. Chem. 90, 396 (1959);
Arch. Pharm. 293, 646 (1960). Stereoisomeric with turicine (
cis-form). Synthesis: Patchett, Witkop,
J. Am. Chem. Soc. 79, 185 (1957).
Properties: Blunt prisms from ethanol, dec 254-256°. Sweet taste. [a]D20 -34.2° (c = 1.0). Readily sol in water or hot alcohol, slightly in cold alcohol. Practically insol in benzene, ether, chloroform, carbon tetrachloride.
Optical Rotation: [a]D20 -34.2° (c = 1.0)
Derivative Type: Hydrochloride
Molecular Formula: C7H13NO3.HCl
Molecular Weight: 195.64
Percent Composition: C 42.97%, H 7.21%, N 7.16%, O 24.53%, Cl 18.12%
Properties: Crystals from ethanol, dec 216-217° (after drying). [a]D20 -24.2° (c = 0.0892 in water).
Optical Rotation: [a]D20 -24.2° (c = 0.0892 in water)
Derivative Type: Aurichloride
Molecular Formula: C7H13NO3.HAuCl4
Molecular Weight: 498.97
Percent Composition: C 16.85%, H 2.83%, N 2.81%, O 9.62%, Au 39.47%, Cl 28.42%
Properties: Scaly clusters from water, mp 242°.
Melting point: mp 242°
Status: This monograph has been retired and is no longer subject to revision or update.