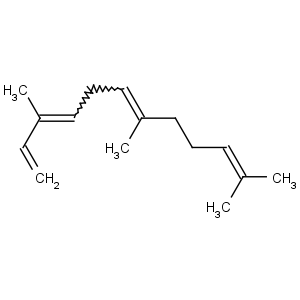
Title: a-Farnesene
CAS Registry Number: 502-61-4
CAS Name: 3,7,11-Trimethyl-1,3,6,10-dodecatetraene
Synonyms: 2,6,10-trimethyl-2,6,9,11-dodecatetraene; farnesene
Molecular Formula: C15H24
Molecular Weight: 204.35
Percent Composition: C 88.16%, H 11.84%
Literature References: Isoln of
(E,E)-form from natural coating of apples: Huelin, Murray,
Nature 210, 1260 (1966); Murray,
Aust. J. Chem. 22, 197 (1969); from Dufour's gland in ants: Cavill
et al., Tetrahedron Lett. 1967, 2201. Isoln of
(Z,E)-form from oil of perilla: T. Sakai, Y. Hirose,
Bull. Chem. Soc. Jpn. 42, 3615 (1969). Oxidation products of farnesene are believed to cause scald, a serious storage disorder of apples. Four possible geometric isomers. Synthetic studies: Ruzicka,
Helv. Chim. Acta 6, 490, 501 (1923); Ruzicka, Capato,
ibid. 8, 267 (1925). Configuration of
(E,E)-form confirmed by synthesis from
trans-b-farnesene: Brieger
et al., J. Org. Chem. 34, 3789 (1969). Stereospecific synthesis of
(E,Z)- and
(Z,Z)-isomers: Anet,
Aust. J. Chem. 23, 2101 (1970). Synthesis of
(E,E)-form: Tanaka
et al., J. Am. Chem. Soc. 97, 3252 (1975).
(E,E)- and
(Z,E)-forms are components of aphid alarm pheromones: J. A. Pickett, D. C. Griffiths,
J. Chem. Ecol. 6, 349 (1980); of the trail pheromone of red imported fire ants: R. K. Vandermeer
et al., Tetrahedron Lett. 22, 1651 (1981).
Properties: Thin oil. bp12 about 125°. d420 0.8410.
nD20 1.4836. Practically insol in water. Misc with hydrocarbon solvents. uv max of
(E,E)-form (alc): 233 nm (e 27,000); of
(Z,E)-form (alc): 238 nm (e 11300).
Boiling point: bp12 about 125°
Index of refraction: nD20 1.4836
Absorption maximum: uv max of
(E,E)-form (alc): 233 nm (e 27,000); of
(Z,E)-form (alc): 238 nm (e 11300)
Density: d420 0.8410