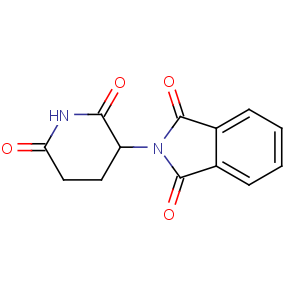
Title: Thalidomide
CAS Registry Number: 50-35-1
CAS Name: 2-(2,6-Dioxo-3-piperidinyl)-1
H-isoindole-1,3(2
H)-dione
Synonyms: N-(2,6-dioxo-3-piperidyl)phthalimide; a-phthalimidoglutarimide; 3-phthalimidoglutarimide; 2,6-dioxo-3-phthalimidopiperidine;
N-phthalylglutamic acid imide;
N-phthaloylglutamimide
Manufacturers' Codes: K-17
Trademarks: Contergan; Neurosedyn; Softenon; Thalomid (Celgene)
Molecular Formula: C13H10N2O4
Molecular Weight: 258.23
Percent Composition: C 60.47%, H 3.90%, N 10.85%, O 24.78%
Literature References: Selective inhibitor of tumor necrosis factor a (TNF-a). Formerly used as sedative, hypnotic. Prepn:
GB 768821 (1957 to Chemie Grünenthal). Teratogenicity studies: I. D. Fratta
et al., Toxicol. Appl. Pharmacol. 7, 268 (1965). Review of proposed mechanisms of embryopathy: T. D. Stephens,
Teratology 38, 229-239 (1988). HPLC determn in plasma: A. Delon
et al., J. Liq. Chromatogr. 18, 297 (1995). Stereospecific determn and pharmacokinetics of enantiomers: T. Eriksson
et al., Chirality 7, 44 (1995). Clinical trial in HIV wasting syndrome: G. Reyes-Terán
et al., AIDS 10, 1501 (1996); in aphthous ulcers related to HIV infection: J. M. Jacobson
et al., N. Engl. J. Med. 336, 1487 (1997). Review of chemistry, pharmacokinetics and clinical safety: V. Günzler,
Drug Saf. 7, 116-134 (1992); of effect on TNF-a and clinical use in leprosy and tuberculosis: J. D. Klausner
et al., Clin. Immunol. Immunopathol. 81, 219-223 (1996); of pharmacology, history and potential clinical uses: D. Stirling
et al., J. Am. Pharm. Assoc. NS37, 307-313 (1997); of use in multiple myeloma: S. V. Rajkumar,
Expert Rev. Anticancer Ther.1, 20-28 (2001); of pharmacology and toxicology: C. Meierhofer, C. J. Wiedermann,
Curr. Opin. Drug Disc. Devel. 6, 92-99 (2003); of clinical experience: S. J. Matthews, C. McCoy,
Clin. Ther. 25, 342-395 (2003).
Properties: Needles, mp 269-271°. uv max (neutral soln): 220, 300 nm. Soly in water: ~2 ′ 10-4 mol/L; 45-60 mg/L. Sparingly sol in water, methanol, ethanol, acetone, ethyl acetate, butyl acetate, glacial acetic acid. Very sol in dioxane, DMF, pyridine. Practically insol in ether, chloroform, benzene.
Melting point: mp 269-271°
Absorption maximum: uv max (neutral soln): 220, 300 nm
Therap-Cat: Immunomodulator.
Keywords: Sedative/Hypnotic; Piperidinediones; Immunomodulator.