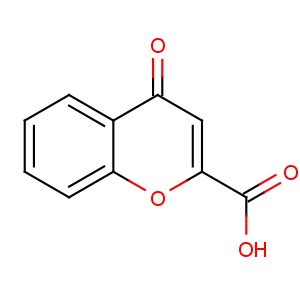
Title: Chromocarb
CAS Registry Number: 4940-39-0
CAS Name: 4-Oxo-4
H-1-benzopyran-2-carboxylic acid
Synonyms: 2-chromonecarboxylic acid; 4-oxo-4
H-chromene-2-carboxylic acid; benzo-g-pyronecarboxylic acid
Molecular Formula: C10H6O4
Molecular Weight: 190.15
Percent Composition: C 63.16%, H 3.18%, O 33.66%
Literature References: Prepn: S. Ruhemann, H. E. Stapleton,
J. Chem. Soc. 77, 1179 (1900); J. Schmutz
et al., Helv. Chim. Acta 34, 767 (1951); G. Pifferi
et al., J. Heterocycl. Chem. 14, 1257 (1977). Prepn of diethylamine salt: P. A. Tronche,
ZA 6807352;
eidem, US 3816470 (1969, 1974 both to Ferlux). Pharmacology of diethylamine salt in animals: J. Couquelet
et al., C.R. Seances Soc. Biol. Ses Fil. 164, 329 (1970); P. Conquet
et al., ibid. 800. Clinical comparison with dipyridamole of effect on platelet function: A. Vittoria
et al., Curr. Ther. Res. 35, 1033 (1984). Clinical trial in diabetes with vascular disease: N. Ciavarella
et al., ibid. 36, 293 (1984). Bioavailability in humans: J.-M. Aiache
et al., Biopharm. Drug Dispos. 7, 301 (1986).
Properties: Colorless needles from alcohol, mp 250-251° (dec) (Ruhemann, Stapleton); also reported as mp 255-256° (Pifferi). uv max: 230, 305 nm (e 20220, 8075). Sol in alcohol, ammonia. Sparingly sol in water.
Melting point: mp 250-251° (dec) (Ruhemann, Stapleton); mp 255-256° (Pifferi)
Absorption maximum: uv max: 230, 305 nm (e 20220, 8075)
Derivative Type: Diethylamine
CAS Registry Number: 23915-80-2
Trademarks: Angiophtal (Merck & Co.); Campel (Farmitalia); Fludarene (Merck & Co.)
Molecular Formula: C14H17NO4
Molecular Weight: 263.29
Percent Composition: C 63.86%, H 6.51%, N 5.32%, O 24.31%
Properties: Microcrystalline powder from alcohol + acetone, mp 138°. Sol in water. LD50 in mice: ~800 mg/kg i.v.; >5 g/kg orally (Tronche, 1974).
Melting point: mp 138°
Toxicity data: LD50 in mice: ~800 mg/kg i.v.; >5 g/kg orally (Tronche, 1974)
Therap-Cat: Diethylamine salt as capillary protectant.
Keywords: Vasoprotectant.