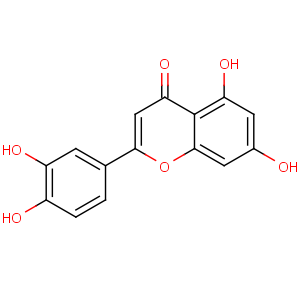
Title: Luteolin
CAS Registry Number: 491-70-3
CAS Name: 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4
H-1-benzopyran-4-one
Synonyms: 3¢,4¢,5,7-tetrahydroxyflavone; digitoflavone; cyanidenon 1470
Molecular Formula: C15H10O6
Molecular Weight: 286.24
Percent Composition: C 62.94%, H 3.52%, O 33.54%
Literature References: Found in many plants in glycosidic combination,
e.g., as the arabinoside: Perkin,
J. Chem. Soc. 69, 800 (1896); Fleischer,
Ber. 32, 1186 (1899); Perkin, Horsfall,
J. Chem. Soc. 77, 1315 (1900); Hayashi, Inoue,
Acta Phytochim. 15, 53 (1949);
C.A. 43, 8450 (1949). Identity with digitoflavone: Kiliani, Mayer,
Ber. 34, 3577 (1901). Synthesis: Hutchins, Wheeler,
J. Chem. Soc. 1939, 91.
See also Bioflavonoids.
Derivative Type: Monohydrate
Properties: Yellow needles from alc, dec 328-330°. Sublimes in high vacuum. Sparingly sol in water. Sol in alkalies forming yellow solns.
Derivative Type: 5-Glucoside
Synonyms: Galuteolin
Molecular Formula: C21H20O11
Molecular Weight: 448.38
Percent Composition: C 56.25%, H 4.50%, O 39.25%
Properties: From seeds of
Galega officinalis L.,
Leguminosae: Barger, White,
Biochem. J. 17, 836 (1923). Structure: Nakamura, Hukuti,
J. Pharm. Soc. Jpn. 60, 449 (1940);
C.A. 34, 79103 (1940); Nordstr?m, Swain,
J. Chem. Soc. 1953, 2764. Yellow needles from hot dil alc, dec 280°. Practically insol in water. Slightly sol in abs alcohol; sol in hot dil alcohol.
Derivative Type: 7-Glucoside
Synonyms: Cynaroside
Molecular Formula: C21H20O11
Molecular Weight: 448.38
Percent Composition: C 56.25%, H 4.50%, O 39.25%
Properties: From
Achillea millefolium L.,
Compositae: H?rhammer
et al., Acta Chim. Acad. Sci. Hung. 40, 463 (1964). Yellow needles from alc, mp 254-256°. uv max (CH3OH): 350, 255 nm (log e 4.30, 4.27).
Melting point: mp 254-256°
Absorption maximum: uv max (CH3OH): 350, 255 nm (log e 4.30, 4.27)