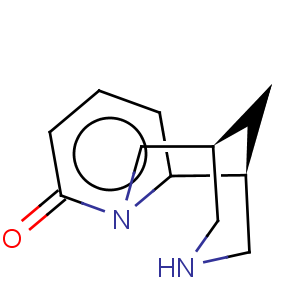
Title: Cytisine
CAS Registry Number: 485-35-8
CAS Name: (1R)-1,2,3,4,5,6-Hexahydro-1,5-methano-8
H-pyrido-[1,2-
a][1,5]diazocin-8-one
Synonyms: baptitoxine; sophorine; ulexine
Trademarks: Cytiton (USSR)
Molecular Formula: C11H14N2O
Molecular Weight: 190.24
Percent Composition: C 69.45%, H 7.42%, N 14.73%, O 8.41%
Literature References: Toxic principle in seed of
Laburnum anagyroides Medik. and other
Leguminosae. Extraction: Ing,
J. Chem. Soc. 1931, 2200; Sp?th, Galinovsky,
Ber. 65, 1526 (1932);
66, 1338 (1933); Lecoq,
Bull. Soc. Chim. Fr. 10, 153 (1943). Structure: Ing,
J. Chem. Soc. 1932, 2778. Synthesis: Bohlmann
et al., Angew. Chem. 67, 708 (1955); Van Tamelen, Baran,
J. Am. Chem. Soc. 77, 4944 (1955). Absolute configuration: Okuda
et al., Chem. Ind. (London) 1961, 1751. Pharmacological properties: R. B. Barlow, L. J. McLeod,
Br. J. Pharmacol. 35, 161 (1969).
Properties: Orthorhombic prisms from acetone, mp 152-153°. Sublimes. bp2 218°. [a]D17 -120°. pK1 6.11; pK2 13.08. Sol in 1.3 parts water, 13 parts acetone, 1.3 parts methanol, 3.5 parts alcohol, 30 parts benzene, 10 parts ethyl acetate, 2.0 parts chloroform. Practically insol in petr ether. LD50 in mice (mg/kg): 1.73 i.v.; 9.4 i.p.; 101 orally (Barlow, McLeod).
Melting point: mp 152-153°
Boiling point: bp2 218°
pKa: pK1 6.11; pK2 13.08
Optical Rotation: [a]D17 -120°
Toxicity data: LD50 in mice (mg/kg): 1.73 i.v.; 9.4 i.p.; 101 orally (Barlow, McLeod)
Derivative Type: Hydrochloride
Molecular Formula: C11H14N2O.HCl
Molecular Weight: 226.70
Percent Composition: C 58.28%, H 6.67%, N 12.36%, O 7.06%, Cl 15.64%
Properties: Deliquescent crystals, sol in water and alcohol. pH of 0.1 molar aq soln 4.3.
Derivative Type: Nitrate monohydrate
Molecular Formula: C11H14N2O.HNO3.H2O
Molecular Weight: 271.27
Percent Composition: C 48.70%, H 6.32%, N 15.49%, O 29.49%
Properties: Needles or leaflets, [a]D -81.5°. Sol in water, slightly sol in alcohol. Practically insol in ether.
Optical Rotation: [a]D -81.5°