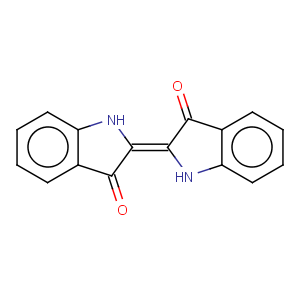
Title: Indigo
CAS Registry Number: 482-89-3
CAS Name: 2-(1,3-Dihydro-3-oxo-2
H-indol-2-ylidene)-1,2-dihydro-3
H-indol-3-one
Synonyms: [D2,2¢-biindoline]-3,3¢-dione; D2,2¢-bipseudoindoxyl; indigotin; indigo blue; D & C Blue No. 6; C.I. Pigment Blue 66; C.I. Vat Blue 1; C.I. 73000
Molecular Formula: C16H10N2O2
Molecular Weight: 262.26
Percent Composition: C 73.28%, H 3.84%, N 10.68%, O 12.20%
Literature References: Probably the oldest known coloring matter. Originally obtained from various species of
Indigofera, Leguminosae, indigenous to Bengal, Java, Guatemala, in which it occurs as a glucoside. First synthesis: Baeyer,
Ber. 11, 1296 (1878);
12, 456 (1879). First commercial process: Heumann,
Ber. 23, 3043, 3431 (1890). Newer prepns: Harley-Mason,
J. Chem. Soc. 1950, 2907; Ziegler, Kappe,
Monatsh. Chem. 96, 889 (1965); J. Gosteli,
Helv. Chim. Acta 60, 1980 (1977). Indigo occurs in
cis and
trans forms, in solid state it is in the
trans form: Posner,
Ber. 56, 31 (1923);
57, 1313 (1924);
59, 1799 (1926). Reviews on indigo and indigoid dyes: Lubs,
The Chemistry of Synthetic Dyes, ACS Monograph Series no.
127 (Reinhold, New York, 1955) pp 551-576; Schweizer,
Künstliche Organische Farbstoffe und ihre Zwischenprodukte (Springer Verlag, 1964) pp 320-334;
Colour Index vol. 4 (3rd ed., 1971) p 4595.
Properties: Dark-blue powder with coppery luster. Sublimes at about 300°; dec 390°. Practically insol in water, alcohol, ether, and dil acids. Dissolves in nonpolar solvents with red and in polar solvents with blue color. With fuming H2SO4 it forms a sol sulfonic acid.
Use: As textile dye. In sutures.