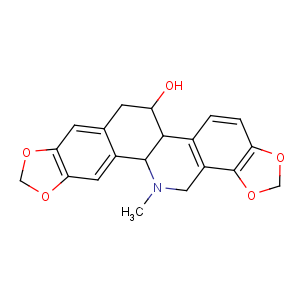
Title: Chelidonine
Synonyms: Stylophorin
Molecular Formula: C20H19NO5
Molecular Weight: 353.37
Percent Composition: C 67.98%, H 5.42%, N 3.96%, O 22.64%
Literature References: Hexahydrobenzophenanthridine alkaloid. Occurs in nature in (+)-form, (-)-form and racemic form. Isoln of (+)-form from root of
Chelidonium majus L.,
Stylophorum diphyllum (Michx.) Nutt., and
Dicranostigma franchetianum (Prain) Fedde,
Papaveraceae: J. M. Probst,
Ann. 29, 113 (1839); E. Schmidt, F. Selle,
Arch. Pharm. 228, 441 (1890); F. Selle,
ibid. 96; Manske,
Can. J. Res. 20B, 53 (1942); J. Slavik,
Collect. Czech. Chem. Commun. 20, 198 (1955); from
Symphoricarpos albus L., Blake,
Caprifoliaceae: M. Szaufer
et al., Phytochemistry 17, 1446 (1978). Isoln of (-)-form from
Glaucium corniculatum Curt.,
Papaveraceae: J. Slavik, L. Slavikova,
Collect. Czech. Chem. Commun. 22, 279 (1957). Structure: F. von Bruchhausen, H. W. Bersch,
Ber. 63, 2520 (1930); E. Sp?th, F. Kuffner,
ibid. 64, 370 (1931); H. W. Bersch,
Arch. Pharm. 291, 491 (1958). Identity of (±)-form with diphylline: J. Slavik,
Collect. Czech. Chem. Commun. 26, 2933 (1961). Absolute configuration of (+)-
p-bromobenzoate: N. Takao
et al., Tetrahedron Lett. 1979, 495. Conformation: M. Cushman, T.-C. Choong,
Heterocycles 14, 1935 (1980); M. Sugiura
et al., J. Chem. Soc. Perkin Trans. 2 1986, 175. Chiroptic properties and absolute configuration of (+)-chelidonine: N. Takao
et al., Arch. Pharm. 317, 223 (1984). Biosynthesis: E. Leete,
J. Am. Chem. Soc. 85, 473 (1963). Total synthesis of (±)-form: W. Oppolzer, K. Keller,
ibid. 93, 3836 (1971); M. Cushman
et al., J. Org. Chem. 45, 5067 (1980); W. Oppolzer, C. Rabbiani,
Helv. Chim. Acta 66, 1119 (1983); M. Hanaoka
et al., Chem. Lett. 1986, 736. Effect on smooth muscle: P. J. Hanzlik:
J. Pharmacol. Exp. Ther. 7, 99 (1915). Inhibition of reverse transcriptase activity: M. L. Sethi,
Can. J. Pharm. Sci. 16, 29 (1981). Cytotoxic effects: M. Cushman
et al., loc. cit. Review of pharmacological effects: V. Preininger, "The Biology of Papaveraceae Alkaloids" in
The Alkaloids vol. 15, R. H. F. Manske, Ed. (Academic Press, Orlando, 1975) pp 241-242. General review: V. Simanek, "Benzophenanthridine Alkaloids"
ibid. vol. 26, A. Brossi, Ed. (1985) pp 185-240. Toxicity data: R. C. Anderson, K. K. Chen,
Fed. Proc. 5, 163 (1946).
Derivative Type: (+)-Form
CAS Registry Number: 476-32-4
Synonyms: [5b
R-(5ba,6b,12ba)]-5b,6,7,12b,13,14-Hexahydro-13-methyl[1,3]benzodioxolo[5,6-
c]-1,3-dioxolo[4,5-
i]phenanthridin-6-ol
Properties: Monoclinic prisms from methanol, ethanol or ethanol + chloroform, mp 135-136°. bp0.002 220° (air-bath temp). [a]D22 +115 ±3° (ethanol); [a]D20 +117° (c = 3 in CHCl3). uv max (methanol): 289, 239, 208 nm (log e 3.89, 3.88, 4.69). Sol in alc, chloroform, ether, amyl alc. Practically insol in water. LD50 in mice (mg/kg): 34.6 ±2.44 i.v. (Anderson, Chen).
Melting point: mp 135-136°
Boiling point: bp0.002 220° (air-bath temp)
Optical Rotation: [a]D22 +115 ±3° (ethanol); [a]D20 +117° (c = 3 in CHCl3)
Absorption maximum: uv max (methanol): 289, 239, 208 nm (log e 3.89, 3.88, 4.69)
Toxicity data: LD50 in mice (mg/kg): 34.6 ±2.44 i.v. (Anderson, Chen)
Derivative Type: (+)-
O-Acetylchelidonine
Molecular Formula: C22H21NO6
Molecular Weight: 395.41
Percent Composition: C 66.83%, H 5.35%, N 3.54%, O 24.28%
Properties: Crystals from chloroform, mp 184-186°. [a]D +110°.
Melting point: mp 184-186°
Optical Rotation: [a]D +110°
Derivative Type: (+)-Benzoylchelidonine
Molecular Formula: C27H23NO6
Molecular Weight: 457.47
Percent Composition: C 70.89%, H 5.07%, N 3.06%, O 20.98%
Properties: Crystals from chloroform, mp 210-211°.
Melting point: mp 210-211°
Derivative Type: (-)-Form
CAS Registry Number: 88200-01-5
Properties: Crystals from aqueous ethanol, mp 136°. [a]D22 -112 ±3° (c = 0.47 in ethanol).
Melting point: mp 136°
Optical Rotation: [a]D22 -112 ±3° (c = 0.47 in ethanol)
Derivative Type: (±)-Form
CAS Registry Number: 20267-87-2
Synonyms: Diphylline
Properties: Crystals from ethanol, mp 215-216°.
Melting point: mp 215-216°