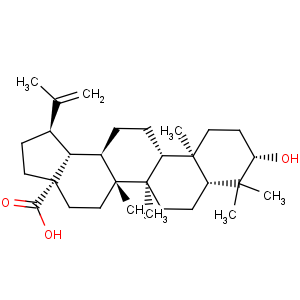
Title: Betulinic Acid
CAS Registry Number: 472-15-1
CAS Name: (3b)-3-Hydroxylup-20(29)-en-28-oic acid
Synonyms: betulic acid
Manufacturers' Codes: ALS-357
Molecular Formula: C30H48O3
Molecular Weight: 456.70
Percent Composition: C 78.90%, H 10.59%, O 10.51%
Literature References: Naturally occurring triterpene found in many plant sources, notably the bark of white birch trees,
Betula alba L.
Betulaceae. Melanoma-specific cytotoxic agent. Isoln: L. Ruzicka
et al., Helv. Chim. Acta 21, 1706 (1938); and characterization: A. Robertson
et al., J. Chem. Soc. 1939, 1267. Synthesis from betulin,
q.v.: D. S. H. Kim
et al., Synth. Commun. 27, 1607 (1997). Isoln and crystal structure of benzyl ester: G. Bringmann
et al., Planta Med. 63, 255 (1997). Inhibition of HIV replication: T. Fujioka
et al., J. Nat. Prod. 57, 243 (1994). Induction of apoptosis in human melanoma cells: E. Pisha
et al., Nat. Med. 1, 1046 (1995). Mechanism of action study: S. Fulda
et al., Cancer Res. 57, 4956 (1997). Pharmacokinetics in mice: G. O. Udeani
et al., Biopharm. Drug Dispos. 20, 379 (1999). Tumor cell-specific cytotoxicity: V. Zuco
et al., Cancer Lett. 175, 17 (2002). LC/MS determn in plasma: X. Cheng
et al., Rapid Commun. Mass Spectrom. 17, 2089 (2003). Review of natural distribution: G. Pavanasasivam, M. U. S. Sultanbawa,
Phytochemistry 13, 2002-2006 (1974); of anticancer activity: D. A. Eiznhamer, Z.-Q. Xu,
IDrugs 7, 359-373 (2004); of chemistry, biology and therapeutic potential: R. H. Cichewicz, S. A. Kouzi,
Med. Res. Rev. 24, 90-114 (2004); of biological properties: P. Yogeeswari, D. Sriram,
Curr. Med. Chem. 12, 657-666 (2005).
Properties: Colorless needles from methanol, mp 290-293°. [a]D25 +7.5° (c = 0.37 in pyridine). Highly sol in pyridine, acetic acid. Limited soly in methanol, ethanol, CHCl3, ether. Low soly in H2O, petroleum ether, DMF, DMSO, benzene.
Melting point: mp 290-293°
Optical Rotation: [a]D25 +7.5° (c = 0.37 in pyridine)