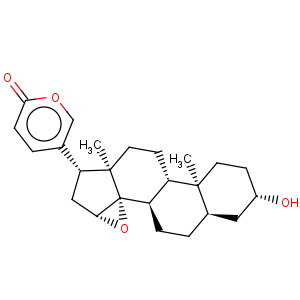
Title: Resibufogenin
CAS Registry Number: 465-39-4
CAS Name: (3b,5b,15b)-14,15-Epoxy-3-hydroxy-5-bufa-20,22-dienolide
Trademarks: Respigon (Taisho)
Molecular Formula: C24H32O4
Molecular Weight: 384.51
Percent Composition: C 74.97%, H 8.39%, O 16.64%
Literature References: Cytotoxic constituent of toad venom. Isoln: Meyer,
Helv. Chim. Acta 35, 2444 (1952); Linde, Meyer,
Pharm. Acta Helv. 33, 327 (1958). Structure: Thiessen,
Chem. Ind. (London) 1958, 440; Linde, Meyer,
Experientia 14, 238 (1958);
eidem, Helv. Chim. Acta 42, 807 (1959). Synthesis: Pettit
et al., J. Org. Chem. 36, 3736 (1971); Haede
et al., Ann. 1973, 5. Stereochemical study of cytotoxic properties: Y. Kamono
et al., J. Chem. Res. Synop. 1977, 78. Pharmacology: Leigh, Caldwell,
J. Pharm. Pharmacol. 21, 708 (1969).
Properties: Crystals from acetone + water, mp 113-140°/155-168°. [a]D22 -7.1° (c = 1.259 in chloroform). Also obtained as an amorphous solid, [a]D16 -5.4° (c = 2.030 in chloroform).
Melting point: mp 113-140°/155-168°
Optical Rotation: [a]D22 -7.1° (c = 1.259 in chloroform); [a]D16 -5.4° (c = 2.030 in chloroform)
Derivative Type: Hydrochloride
Molecular Formula: C24H33ClO4
Molecular Weight: 420.97
Percent Composition: C 68.47%, H 7.90%, Cl 8.42%, O 15.20%
Properties: Crystals from acetone, dec 230-232°. [a]D15 +15.1° (c = 0.5302 in chloroform). uv max (alc): 298 nm (log e 3.74).
Optical Rotation: [a]D15 +15.1° (c = 0.5302 in chloroform)
Absorption maximum: uv max (alc): 298 nm (log e 3.74)
Therap-Cat: Cardiotonic.
Keywords: Cardiotonic.