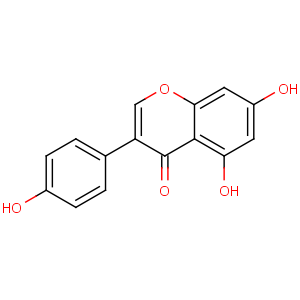
Title: Genistein
CAS Registry Number: 446-72-0
CAS Name: 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4
H-1-benzopyran-4-one
Synonyms: 4¢,5,7-trihydroxyisoflavone; prunetol; genisteol
Molecular Formula: C15H10O5
Molecular Weight: 270.24
Percent Composition: C 66.67%, H 3.73%, O 29.60%
Literature References: Phytoestrogen found in soy products; the aglucon of genistin and of sophoricoside. Specific protein kinase inhibitor. Prepn from the glucoside by hydrolysis with emulsin: Charaux, Rabaté,
J. Pharm. Chim. [9]
1, 404 (1941); by hydrolysis with HCl in methanol: Walter,
J. Am. Chem. Soc. 63, 3273 (1941). Isoln from
prunus spp.,
Rosaceae: Hasegawa
ibid. 79, 1738 (1957); from
Podocarpus spicata R.Br.,
Podocarpaceae: Briggs, Cebalo,
Tetrahedron 6, 145 (1959). Structure: Baker, Robinson,
J. Chem. Soc. 1925, 1981;
1926, 2713; Walz,
Ann. 489, 118 (1931). Synthesis: Baker, Robinson,
J. Chem. Soc. 1928, 3115; Narasimhachari
et al., J. Sci. Ind. Res. 12, 287 (1953); Yoder
et al., Proc. Iowa Acad. Sci. 61, 271 (1954); Zemplén
et al., Acta Chim. Acad. Sci. Hung. 19, 277 (1959). HPLC determn in biological fluids: J. G. Supko, L. R. Phillips,
J. Chromatogr. B 666, 157 (1995); A. A. Franke
et al, Proc. Soc. Exp. Biol. Med. 208, 18 (1995). Review of synthesis and isotopic labeling: K. W?h?l?
et al., ibid. 27-32. Series of articles on chemopreventive properties and mechanism of action:
ibid. 103-115, 120-130;
J. Nutr. 125, Suppl. 3, 777S-797S (1995).
Properties: Rectangular or six-sided rods from 60% alcohol. Dendritic needles from ether. mp 297-298° (slight decompn). Sol in the usual organic solvents, in dil alkalies with yellow color. Practically insol in water. uv max: 262.5 nm (e 138).
Melting point: mp 297-298° (slight decompn)
Absorption maximum: uv max: 262.5 nm (e 138)
Derivative Type: Sophoricoside
CAS Registry Number: 152-95-4
Synonyms: Genistein-4¢-glucoside
Molecular Formula: C21H20O10
Molecular Weight: 432.38
Percent Composition: C 58.33%, H 4.66%, O 37.00%
Literature References: From the green pods of
Sophora japonica L.,
Leguminosae: Charaux, Rabate,
Bull. Soc. Chim. Biol. 20, 454 (1938). Structure: Zemplén
et al., Ber. 76, 267 (1943). Synthesis: Bognár, Szabo,
Acta Chim. Acad. Sci. Hung. 4, 383 (1954);
Chem. Ind. (London) 1954, 518.
Properties: Crystals from alcohol, mp 298°. [a]D20 -47° (pyridine). [a]D20 -32° (10% aq pyridine). uv max (abs ethanol): 262 nm. Sparingly sol in water, alc, acetic acid; more sol in hot alc, hot acetic acid; sol in pyridine, dil alkalies. Practically insol in ethyl acetate, acetone.
Melting point: mp 298°
Optical Rotation: [a]D20 -47° (pyridine); [a]D20 -32° (10% aq pyridine)
Absorption maximum: uv max (abs ethanol): 262 nm
Derivative Type: Genistin
CAS Registry Number: 529-59-9
Synonyms: Genistein-7-
O-b-D-glucoside
Molecular Formula: C21H20O10
Molecular Weight: 432.38
Percent Composition: C 58.33%, H 4.66%, O 37.00%
Literature References: For isoln and structure
see Walter, Hasegawa, Walz,
loc. cit. Synthesis: Zemplén, Farkas,
Ber. 76B, 1110 (1943).
Properties: Pale yellow plates from 80% ethanol, mp 256°. [a]D21 -28° (c = 0.6 in 0.02
N NaOH); [a]D26 -21.4° (pyridine). uv max (85% ethanol): 262.5 nm (a 90.5). Practically insol in cold water. Slightly sol in hot water, hot ethanol, hot methanol; sol in hot 80% ethanol, hot 80% methanol, hot acetone, pyridine.
Melting point: mp 256°
Optical Rotation: [a]D21 -28° (c = 0.6 in 0.02
N NaOH); [a]D26 -21.4° (pyridine)
Absorption maximum: uv max (85% ethanol): 262.5 nm (a 90.5)
Derivative Type: Olmelin
Synonyms: 5,7-Dihydroxy-4¢-methoxyisoflavone; biochanin A; 4¢-methyl ether
Molecular Formula: C16H12O5
Molecular Weight: 284.26
Percent Composition: C 67.60%, H 4.26%, O 28.14%
Literature References: Isoln from red clover: Pope
et al., Chem. Ind. (London) 1953, 1092; Wong,
J. Sci. Food Agric. 13, 304 (1962); from
Andira inermis (Swartz) H.B.K.,
Leguminosae: Crocker
et al., J. Chem. Soc. 1962, 4906. Identity with olmelin: Gakhokidze,
J. Appl. Chem. USSR 23, 789 (1950),
C.A. 46, 9098i (1952). Structure: Bose, Siddiqui,
J. Sci. Ind. Res. 9B, no. 1, 25 (1950). Synthesis: Baker
et al., Nature 169, 706 (1952).
Properties: Yellow needles from methanol, mp 212-216°.
Melting point: mp 212-216°
Use: Chemical probe to explore signal transduction pathways.