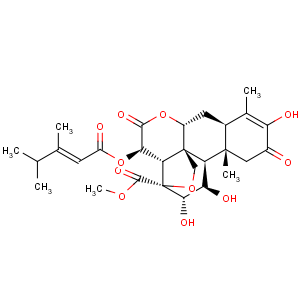
Title: Bruceantin
CAS Registry Number: 41451-75-6
CAS Name: [11b,12a,15b(
E)]-15-[(3,4-Dimethyl-1-oxo-2-pentenyl)oxy]-13,20-epoxy-3,11,12-trihydroxy-2,16-dioxopicras-3-en-21-oic acid methyl ester
Manufacturers' Codes: NSC-165563
Molecular Formula: C28H36O11
Molecular Weight: 548.58
Percent Composition: C 61.30%, H 6.61%, O 32.08%
Literature References: Antileukemic quassinoid from the simaroubaceous tree
Brucea antidysenterica J. F. Mill. Isoln and structure: S. M. Kupchan,
DE 2347576; S. M. Kupchan, R. W. Britton,
US 3969369 (1975, 1976 both to Research Corp.); S. M. Kupchan
et al., J. Org. Chem. 40, 648 (1975). Mode of action: L. L. Liao
et al., Mol. Pharmacol. 12, 167 (1976).
In vivo study: R. K. Johnson
et al., Cancer Treat. Rep. 62, 1535 (1978). Pharmacology: S. M. Sieber
et al., ibid. 60, 1127 (1976); M. Fresno
et al., Biochim. Biophys. Acta 518, 104 (1978). Clinical study: A. Y. Bedikian
et al., Proc. Am. Assoc. Cancer Res. 20, 193 (1979). Toxicologic evaluation: T. R. Castles
et al., U.S. NTIS Report PB-257175 (1976) 348 pp. Synthetic studies: O. D. Dailey, P. L. Fuchs,
J. Org. Chem. 45, 216 (1980); R. J. Pariza, P. L. Fuchs,
ibid. 48, 2306 (1983). Total synthesis: M. Sasaki
et al., ibid. 55, 528 (1990).
Properties: Crystals from ether, mp 225-226°. [a]D25 -43° (c = 0.31 in pyridine). uv max (ethanol): 280, 221 nm (e 8680, 18000); (ethanol, NaOH): 328, 221 nm (e 7290, 28600). LD50 in male, female mice (mg/kg): 1.95, 2.58 i.v. (Castles).
Melting point: mp 225-226°
Optical Rotation: [a]D25 -43° (c = 0.31 in pyridine)
Absorption maximum: uv max (ethanol): 280, 221 nm (e 8680, 18000); (ethanol, NaOH): 328, 221 nm (e 7290, 28600)
Toxicity data: LD50 in male, female mice (mg/kg): 1.95, 2.58 i.v. (Castles)