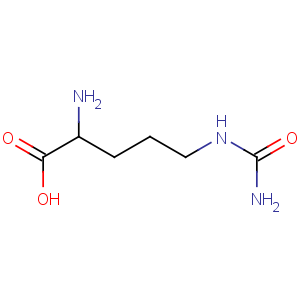
Title: Citrulline
CAS Registry Number: 372-75-8
CAS Name: N5-(Aminocarbonyl)-L-ornithine
Synonyms: d-ureidonorvaline; a-amino-d-ureidovaleric acid;
Nd-carbamylornithine
Molecular Formula: C6H13N3O3
Molecular Weight: 175.19
Percent Composition: C 41.13%, H 7.48%, N 23.99%, O 27.40%
Line Formula: H2NCONH(CH2)3CH(NH2)COOH
Literature References: An amino acid, first isolated from the juice of watermelon,
Citrullus vulgaris Schrad.,
Cucurbitaceae: Wada,
Biochem. Z. 224, 420 (1930); isoln from casein: Wada,
ibid. 257, 1 (1933). Synthesis from ornithine through copper complexes: Kurtz,
J. Biol. Chem. 122, 477 (1938); by alkaline hydrolysis of arginine: Fox,
ibid. 123, 687 (1938); from cyclopentanone oxime: Fox
et al., J. Org. Chem. 6, 410 (1941). Crystallization: Matsuda
et al., JP 71 174 (1971 to Ajinomoto),
C.A. 74, 126056u (1971). Crystal and molecular structure: Naganathan, Venkatesan,
Acta Crystallogr. 27B, 1079 (1971); Ashida
et al., ibid. 28B, 1367 (1972). Use in asthenia and hepatic insufficiency:
FR 2198739 (1974 to Hublot & Vallet),
C.A. 82, 144952c (1975). Clinical trial in treatment of lysinuric protein intolerance: J. Rajantie
et al., J. Pediatr. 97, 927 (1980); T. O. Carpenter
et al., N. Engl. J. Med. 312, 290 (1985).
Properties: Prisms from methanol + water, mp 222°. [a]D20 +3.7° (c = 2). pK1 2.43; pK2 9.41. Sol in water. Insol in methanol, ethanol.
Melting point: mp 222°
pKa: pK1 2.43; pK2 9.41
Optical Rotation: [a]D20 +3.7° (c = 2)
Derivative Type: Hydrochloride
CAS Registry Number: 34312-10-2
Molecular Formula: C6H13N3O3.HCl
Molecular Weight: 211.65
Percent Composition: C 34.05%, H 6.67%, N 19.85%, O 22.68%, Cl 16.75%
Properties: Crystals, dec 185°. [a]D22 +17.9° (c = 2).
Optical Rotation: [a]D22 +17.9° (c = 2)
Derivative Type: Malate (salt)
CAS Registry Number: 54940-97-5
Trademarks: Stimol (Biocodex)
Molecular Formula: C6H13N3O3.C4H6O5
Molecular Weight: 309.27
Percent Composition: C 38.84%, H 6.19%, N 13.59%, O 41.39%
Therap-Cat: Treatment of asthenia.