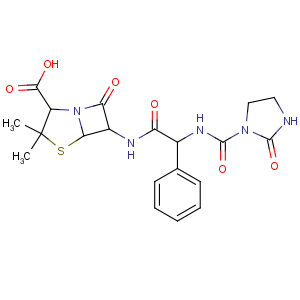
Title: Azlocillin
CAS Registry Number: 37091-66-0
CAS Name: (2
S,5
R,6
R)-3,3-Dimethyl-7-oxo-6-[[(2
R)-[[(2-oxo-1-imidazolidinyl)carbonyl]amino]phenylacetyl]amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
Synonyms: D-a-[(imidazolidin-2-on-1-yl)carbonylamino]benzylpenicillin
Manufacturers' Codes: Bay e 6905
Molecular Formula: C20H23N5O6S
Molecular Weight: 461.49
Percent Composition: C 52.05%, H 5.02%, N 15.18%, O 20.80%, S 6.95%
Literature References: Semi-synthetic, broad-spectrum acylureido penicillin. Prepn: H. Disselnkotter, K. G. Metzger,
FR 2100682;
eidem, US 3933795 (1971, 1976 both to Bayer); H. B. Konig
et al., Eur. J. Med. Chem. - Chim. Ther. 17, 59 (1982).
In vitro studies: D. Stewart
et al., Antimicrob. Agents Chemother. 11, 865 (1977).
In vitro and
in vivo activity: G. K. Daikos
et al., Curr. Chemother., Proc. 10th Int. Congr. Chemother., 1977 (Amer. Soc. Microbiol., Washington, D.C., 1978)
1, pp 626-8. Pharmacokinetics: P. Fiegel, K. Becker,
Antimicrob. Agents Chemother. 14, 288 (1978). Comparison with other penicillins: J. M. Andrews, K. A. Bedford,
ibid. 559. Clinical studies: H. Lode
et al., Infection 5, 163 (1977); E. B. Helm
et al., Dtsch. Med. Wochenschr. 102, 1211 (1977). Series of articles on antibacterial activity, pharmacology, and clinical trials:
Arzneim.-Forsch. 29, 1915-2032 (1979);
Infection 10, Suppl. 3, S121-S266 (1982);
J. Antimicrob. Chemother. 11, Suppl. B, 1-239 (1983).
Derivative Type: Sodium salt
CAS Registry Number: 37091-65-9
Trademarks: Azlin (Bayer); Securopen (Bayer)
Molecular Formula: C20H22N5NaO6S
Molecular Weight: 483.47
Percent Composition: C 49.69%, H 4.59%, N 14.49%, Na 4.76%, O 19.86%, S 6.63%
Properties: Pale yellow crystals, sol in water, methanol, DMF. Slightly sol in ethanol, isopropanol.
Therap-Cat: Antibacterial.
Keywords: Antibacterial (Antibiotics); ?Lactams; Penicillins.