Title: Ecdysteroids
Literature References: Polyhydroxylated steroids formerly known as ecdysones. Originally identified as insect molting hormones controlling the pupation of insects. Later shown to be involved in many complex developmental processes in metamorphosis, differentiation and reproduction. Ecdysteroids have been detected in invertebrate species of several phylla belonging to the Protostomia and in some plant species. The terms
zooecdysteroids and
phytoecdysteroids are used to distinguish ecdysteroids isolated from animal species from those of plant origin. Nomenclature: T. W. Goodwin
et al., Nature 272, 122 (1978). The two major ecdysteroids isolated are ecdysone and 20-hydroxyecdysone. Configuration: M. Koreeda
et al., J. Am. Chem. Soc. 93, 4084 (1971). Chromosomal action: M. Ashburner,
Nature 285, 435 (1980). GC and HPLC determn: R. P. Evershed
et al., J. Chromatogr. 390, 357 (1987).
Reviews: Kilby,
Discovery 18, 13 (1957); P. Karlson,
Angew. Chem. Int. Ed. 2, 175-182 (1963); K. Nakanishi,
Pure Appl. Chem. 25, 167-195 (1971); M. Koreeda, B. A. Teicher,
Anal. Biochem. Insects 1977, 207-240; P. Karlson,
Dev. Endocrinol. 7, 1-11 (1980).
Book: Ecdysone: From Chemistry to Mode of Action, J. Koolman, Ed. (Thieme, New York, 1989) 482 pp.
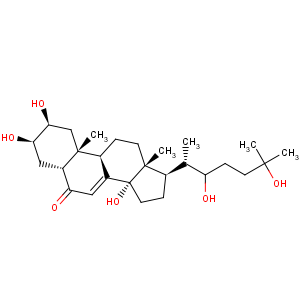
Derivative Type: Ecdysone
CAS Registry Number: 3604-87-3
CAS Name: (2b,3b,5b,22
R)-2,3,14,22,25-Pentahydroxycholest-7-en-6-one
Synonyms: a-ecdysone
Molecular Formula: C27H44O6
Molecular Weight: 464.63
Percent Composition: C 69.80%, H 9.55%, O 20.66%
Literature References: Secreted by ecdysial tissues, then transformed to more active compound, 20-hydroxyecdysone. First isoln from silkworm moths,
Bombyx mori: A. Butenandt, P. Karlson,
Z. Naturforsch. 9b, 389 (1954). Isoln from rhizomes of
Polypodium vulgare L.: G. Heinrich, H. Hoffmeister,
Experientia 23, 995 (1967); from bracken fern,
Pteridinium aquilinum: J. N. Kaplanis
et al., Science 157, 1436 (1967). Structure: P. Karlson
et al., Ber. 98, 2394 (1965). Configuration: R. Huber, W. Hoppe,
ibid. 2403. Synthesis: U. Kerb
et al., Helv. Chim. Acta 49, 1601 (1966); J. B. Siddall
et al., J. Am. Chem. Soc. 88, 379, 862 (1966); H. Mori
et al., Chem. Pharm. Bull. 16, 563 (1968).
Properties: mp 238-239°. [a]20578 +62°. uv max: 243 nm (e 11600).
Melting point: mp 238-239°
Optical Rotation: [a]20578 +62°
Absorption maximum: uv max: 243 nm (e 11600)
Derivative Type: 20-Hydroxyecdysone
CAS Registry Number: 5289-74-7
CAS Name: (2b,3b,5b,22
R,)-2,3,14,20,22,25-Hexahydroxycholest-7-en-6-one
Synonyms: b-ecdysone; ecdysterone; crustecdysone; isoinokosterone; polypodine A
Molecular Formula: C27H44O7
Molecular Weight: 480.63
Percent Composition: C 67.47%, H 9.23%, O 23.30%
Literature References: Most widely occurring ecdysteroid in both plant and animal species. Isoln from
B. mori: P. Hocks, R. Wiechert,
Tetrahedron Lett. 1966, 2989; from seawater crayfish,
Jasus lalandei: F. Hampshire, D. H. S. Horn,
Chem. Commun. 1966, 37. Isolns from plant sources,
Achyranthes fauriei: T. Takemoto
et al., Yakugaku Zasshi 87, 325 (1967);
P. elatus: M. N. Galbraith, D. H. S. Horn,
Chem. Commun. 1966, 905;
P. vulgare: J. Jizba
et al., Tetrahedron Lett. 1967, 1689. Isoln from parasitic helminths: H. H. Rees, J. G. Mercer,
Adv. Invertebr. Reprod. 4, 173 (1986). Configuration: Dammeier, Hoppe,
Ber. 104, 1660 (1971). Synthesis: G. Hüppi, J. B. Siddall,
J. Am. Chem. Soc. 89, 6790 (1967); U. Kerb
et al., Tetrahedron Lett. 1968, 4277; H. Mori, K. Shibata,
Chem. Pharm. Bull. 17, 1970 (1969). Total synthesis: T. Kametani
et al., Tetrahedron Lett. 21, 4855 (1980).
Properties: From methanol-ethyl acetate, mp 240-242°. uv max: 243 nm (e 10300). Unstable in alkaline soln.
Melting point: mp 240-242°
Absorption maximum: uv max: 243 nm (e 10300)