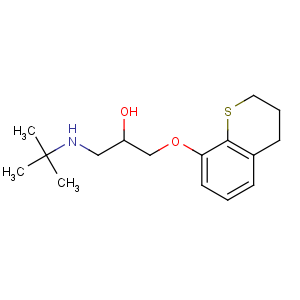
References of 1-(tert-butylamino)-3-(3,4-dihydro-2H-thiochromen-8-yloxy)propan-2-ol
Title: Tertatolol
CAS Registry Number: 34784-64-0
CAS Name: 1-[(3,4-Dihydro-2
H-1-benzothiopyran-8-yl)oxy]-3-[(1,1-dimethylethyl)amino]-2-propanol
Synonyms: (±)-1-
tert-butylamino-3-(1-thiachroman-8-yloxy)-2-propanol;
dl-8-[2-hydroxy-3-[(
tert-butylamino)propyl]oxy]thiochromane
Molecular Formula: C16H25NO2S
Molecular Weight: 295.44
Percent Composition: C 65.05%, H 8.53%, N 4.74%, O 10.83%, S 10.85%
Literature References: Nonselective b-adrenergic blocker. Prepn: C. Malen, M. Laubie,
DE 2115201;
eidem, US 3960891 (1971, 1976 both to Sci. Union et Cie). Pharmacology in animals: M. Laubie
et al., Arch. Int. Pharmacodyn. Ther. 201, 323, 334 (1973); B. R. Walker
et al., J. Cardiovasc. Pharmacol. 7, 1193 (1985). Synergistic effect with indapamide,
q.v.: E. Marmo
et al., Drugs Exp. Clin. Res. 11, 709 (1985). GC-MS determn in plasma and urine: S. Staveris
et al., J. Chromatogr. 339, 97 (1985). Pharmacology in humans and mechanism of action study: A. De Blasi
et al., Clin. Pharmacol. Ther. 39, 245 (1986). Preliminary evaluation in hypertension: J. P. Degaute
et al., Am. J. Hypertens. 1, 263S (1988).
Properties: Crystals from hexane, mp 70-72°.
Melting point: mp 70-72°
Derivative Type: Hydrochloride
CAS Registry Number: 33580-30-2
Manufacturers' Codes: S-2395; SE-2395
Trademarks: Artex (Servier); Artexal (Servier); Prenalex (Itherapia)
Molecular Formula: C16H25NO2S.HCl
Molecular Weight: 331.90
Percent Composition: C 57.90%, H 7.90%, N 4.22%, O 9.64%, S 9.66%, Cl 10.68%
Properties: Crystals from acetonitrile, mp 180-183°. pKa 9.8. LD50 in rats, mice (mg/kg): 40, 37 i.v.; 90, 120 i.p. (Laubie).
Melting point: mp 180-183°
pKa: pKa 9.8
Toxicity data: LD50 in rats, mice (mg/kg): 40, 37 i.v.; 90, 120 i.p. (Laubie)
Therap-Cat: Antihypertensive.
Keywords: ?Adrenergic Blocker; Antihypertensive; Aryloxypropanolamine Derivatives.