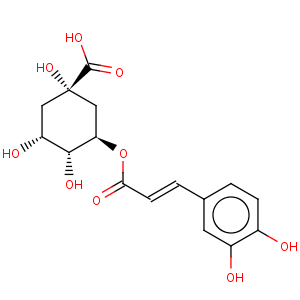
Title: Chlorogenic Acid
CAS Registry Number: 327-97-9
CAS Name: [1
S-(1a,3b,4a,5a)]-3-[[3-(3,4-Dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-1,4,5-trihydroxycyclohexanecarboxylic acid
Synonyms: 1,3,4,5-tetrahydroxycyclohexanecarboxylic acid 3-(3,4-dihydroxycinnamate); 3-caffeoylquinic acid; 3-(3,4-dihydroxycinnamoyl)quinic acid
Molecular Formula: C16H18O9
Molecular Weight: 354.31
Percent Composition: C 54.24%, H 5.12%, O 40.64%
Literature References: Important factor in plant metabolism. Isoln from green coffee beans: Freudenberg,
Ber. 53, 237 (1920). Chlorogenic acid and its isomers isochlorogenic acid and neochlorogenic acid occur also in fruit, leaves and other tissues of dicotyledenous plants: Sondheimer,
Arch. Pharm. 293, 721 (1960). Forms caffeic acid on hydrolysis: Fiedler,
Arzneim.-Forsch. 4, 41 (1954). Structure: Fischer, Dangschat,
Ber. 65, 1037 (1932); Barnes
et al., J. Am. Chem. Soc. 72, 4178 (1950); Corse
et al., Tetrahedron 18, 1207 (1962). Synthesis: Panizzi
et al., Gazz. Chim. Ital. 86, 913 (1956).
Derivative Type: Hemihydrate
Properties: Needles from water. Becomes anhydr at 110°. mp 208°. [a]D26 -35.2° (c = 2.8). pKa (27°) 2.66. R
f values: Fiedler,
loc. cit. Soly in water at 25° about 4%, much more sol in hot water. Alkaline solns acquire an orange color. Freely sol in alcohol, acetone. Very slightly sol in ethyl acetate. Heating with dil HCl yields caffeic acid. Forms a black compd with iron, said to be responsible for the blackening of cut and cooked potatoes:
Chem. Ind. (London) 1958, 627.
Melting point: mp 208°
pKa: pKa (27°) 2.66
Optical Rotation: [a]D26 -35.2° (c = 2.8)
Derivative Type: 3¢-Methyl ether
Synonyms: 3-Feruloylquinic acid
Molecular Formula: C17H20O9
Molecular Weight: 368.34
Percent Composition: C 55.43%, H 5.47%, O 39.09%
Properties: Crystals from ethyl acetate + petr ether, mp 196-197°. [a]D25 -42.8° (ethanol). uv max (ethanol): 325 nm (e 19,200).
Melting point: mp 196-197°
Optical Rotation: [a]D25 -42.8° (ethanol)
Absorption maximum: uv max (ethanol): 325 nm (e 19,200)