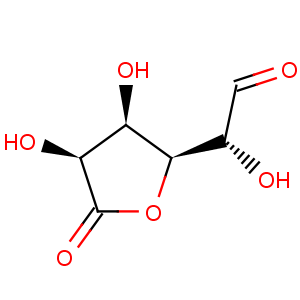
Title: D-Glucuronolactone
CAS Registry Number: 32449-92-6
CAS Name: D-Glucuronic acid g-lactone
Synonyms: D-glucofuranurono-6,3-lactone; glucurolactone; glucurone
Trademarks: Dicurone; Glucoxy; Guronsan
Molecular Formula: C6H8O6
Molecular Weight: 176.12
Percent Composition: C 40.92%, H 4.58%, O 54.51%
Literature References: Found in many plant gums in polymeric combination with other carbohydrates. Important structural constituent of practically all fibrous and connective tissues in the animal organism,
cf. D-glucuronic acid. Prepd synthetically from many polysaccharides or suitable glucosides where the hydroxyl at carbon 6 may be oxidized while the other sensitive groups are protected. Prepn: Stacey,
J. Chem. Soc. 1939, 1529; Hardegger, Spitz,
Helv. Chim. Acta 33, 337 (1950); Marsh,
Proc. Biochem. Soc. [
Biochem. J.],
50, XI (1951); Mehltretter
et al., J. Am. Chem. Soc. 73, 2424 (1951); Phillips, Moody,
J. Chem. Soc. 1960, 762. Structure: J. Stanek
et al., The Monosaccharides (Academic Press, New York, 1963) p 259. For isoln procedures see the ref under glucuronic acid.
Properties: Crystals from ethanol, mp 176-178°. (Commercial grades, mp 172°.) d430 1.76. [a]D25 +19.8° (c = 5.19). Soluble in water (26.9 g/100 ml of soln); slightly sol in methanol (2.8 g/100 ml). Very slightly sol in abs ethanol (0.7 g/100 ml), in glacial acetic acid (0.3 g/100 ml). The free acid is more sol than the lactone. At room temp an aq soln of glucuronolactone reaches an equilibrium of about 20% lactone and 80% acid within 2 months. At 100° an equilibrium of 60% lactone and 40% free acid is reached within 2 hrs. Initial pH of 10% aq soln 3.5, after 1 week the pH is about 2.5.
Melting point: mp 176-178°; Commercial grades, mp 172°
Optical Rotation: [a]D25 +19.8° (c = 5.19)
Density: d430 1.76
Therap-Cat: Detoxicant.