References of 4-Pyrimidinecarboxylicacid, 1,2,3,6-tetrahydro-2,6-dioxo-3-b-D-ribofuranosyl-
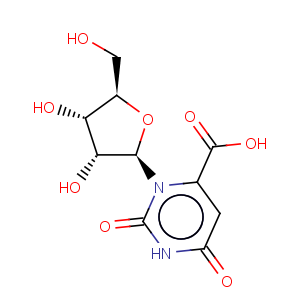
Title: Orotidine
CAS Registry Number: 314-50-1
CAS Name: 1,2,3,6-Tetrahydro-2,6-dioxo-3-b-D-ribofuranosyl-4-pyrimidinecarboxylic acid
Synonyms: 3-b-D-ribofuranosylorotic acid; 6-carboxyuridine
Molecular Formula: C10H12N2O8
Molecular Weight: 288.21
Percent Composition: C 41.67%, H 4.20%, N 9.72%, O 44.41%
Literature References: An orotic acid riboside obtained from cultures of
Neurospora crassa mutants: Michelson
et al., Proc. Natl. Acad. Sci. USA 37, 396 (1951). Isoln: Mitchell, Michelson,
US 2788346 (1957 to California Inst. Res. Found.). Structure: Fox
et al., Biochim. Biophys. Acta 23, 295 (1957). Synthesis: Curran, Angier,
J. Org. Chem. 31, 201 (1966);
US 3282919 (1966 to Am. Cyanamid). Synthesis of orotidine 5¢-phosphate: Moffatt,
J. Am. Chem. Soc. 85, 1118 (1963).
Properties: Needles from methanol + benzene. Turned brown near 200° but failed to melt at 400°. uv max (methanol): 268 nm (e 8900); in 0.1
N HCl: 267 nm (e 9570); in 0.1
N methanolic NaOH: 265 nm (e 8960). Soluble in hot water, lower aliphatic alcohols and aq solns of such alcohols.
Absorption maximum: uv max (methanol): 268 nm (e 8900); in 0.1
N HCl: 267 nm (e 9570); in 0.1
N methanolic NaOH: 265 nm (e 8960)
Derivative Type: Cyclohexamine salt
Molecular Formula: C16H25N3O8
Molecular Weight: 387.39
Percent Composition: C 49.61%, H 6.50%, N 10.85%, O 33.04%
Properties: Crystals from ethanol + benzene, mp 183-184°. [a]D +15° (c = 1). Also isolated as the lead salt.
Melting point: mp 183-184°
Optical Rotation: [a]D +15° (c = 1)
Derivative Type: Orotidine 5¢-phosphate trisodium salt trihydrate
Molecular Formula: C10H10N2O11PNa3.3H2O
Molecular Weight: 488.18
Percent Composition: C 24.60%, H 3.30%, N 5.74%, O 45.88%, P 6.34%, Na 14.13%
Properties: uv max (0.1
N HCl): 267 nm (e 9430). Soluble in water.
Absorption maximum: uv max (0.1
N HCl): 267 nm (e 9430)
Derivative Type: N3-Methylorotidine methyl ester
Molecular Formula: C12H16N2O8
Molecular Weight: 316.26
Percent Composition: C 45.57%, H 5.10%, N 8.86%, O 40.47%
Properties: Stout crystals from isopropanol, mp 135-137°. uv max (methanol): 271 nm (e 7620).
Melting point: mp 135-137°
Absorption maximum: uv max (methanol): 271 nm (e 7620)