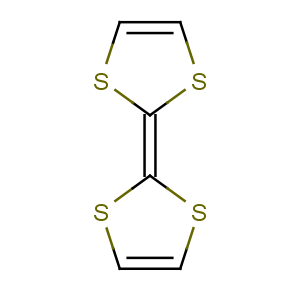
Title: Tetrathiafulvalene
CAS Registry Number: 31366-25-3
CAS Name: 2-(1,3-Dithiol-2-ylidene)-1,3-dithiole
Synonyms: D 2,2¢-bi-1,3-dithiole; bis-1,3-dithiole; 1,4,5,8-tetrathiafulvalene; TTF
Molecular Formula: C6H4S4
Molecular Weight: 204.36
Percent Composition: C 35.26%, H 1.97%, S 62.76%
Literature References: Of interest in solid state chemistry as a conductor, catalyst or sensor due to its unusual electronic and magnetic properties. Primarily used as the parent compound for supramolecular assemblies. Prepn: F. Wudl
et al., Chem. Commun. 1970, 1453. In combination with TCNQ (
tetracyano-p-quinodimethane) to form first "organic metal": J. Ferraris
et al., J. Am. Chem. Soc. 95, 948 (1973); electronic structure: R. Gleiter
et al., J. Electron. Spectrosc. Relat. Phenom. 2, 207 (1973). Use as a catalyst in radical reactions: R. J. Fletcher
et al., J. Chem. Soc. Perkin Trans. 1 1995, 623; J. A. Murphy, S. J. Roome,
ibid. 1349. TTF-mediated biosensors for glucose: T. Yu
et al., J. Appl. Polym. Sci. 58, 973 (1995); for NADH: X. Zhang
et al., Anal. Commun. 33, 111 (1996); for gln and glu: A. Mulchandani, A. S. Bassi,
Biosens. Bioelectron. 11, 271 (1996).
Review: T. Jorgensen
et al., Chem. Soc. Rev. 23, 41-51 (1994). Review of electrical properties and structural data of unsymmetric TTF cmpds: J. M. Fabre
et al., Synth. Met. 35, 57-64 (1990); of role in superconductors: M. R. Bryce,
J. Mater. Chem. 5, 1481-1496 (1995).
Properties: Orange solid in its neutral state; loses electrons to form purple cation radical and subsequent yellow dication. Also reported as yellow solid, mp 118.5-119°. Sublimes 100°, 0.3 mm. uv max (CH2Cl2): 290, 310 nm (e 4 ′ 104, 4 ′ 104). Insol in water. Readily photo-oxidized in air to violet water-soluble radical cation. The cation is a deep purple crystalline solid, mp 155-165° (dec). uv max (H2O): 250 nm (e 4.7 ′ 104); absorption max (H2O): 340, 405, 435, 575 nm (e 4.4 ′ 104; 3.6 ′ 104; 5.6 ′ 104; 1.6 ′ 104). A counter ion is necessary to prevent further oxidation to the dication. Ethanolic solutions of the cation are stable for prolonged periods of time (>48hr).
Melting point: mp 118.5-119°; mp 155-165° (dec)
Absorption maximum: uv max (CH2Cl2): 290, 310 nm (e 4 ′ 104, 4 ′ 104); uv max (H2O): 250 nm (e 4.7 ′ 104); absorption max (H2O): 340, 405, 435, 575 nm (e 4.4 ′ 104; 3.6 ′ 104; 5.6 ′ 104; 1.6 ′ 104)
Use: Molecular sensors; radical catalyst.