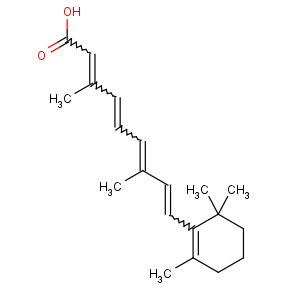
References of (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,
8-tetraenoic acid
Title: Retinoic Acid
CAS Registry Number: 302-79-4
Synonyms: (
all-
E)-3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid;
all-trans-retinoic acid; vitamin A acid; tretinoin
Trademarks: Aberel (Janssen-Cilag); Airol (Pierre Fabre); Avita (Bertek); Epi-Aberel (Janssen-Cilag); Eudyna (BASF); Kerlocal (Pierre Fabre); Renova (Ortho); Retin-A (Ortho); Retinova (Janssen-Cilag); Vesanoid (Roche)
Molecular Formula: C20H28O2
Molecular Weight: 300.44
Percent Composition: C 79.95%, H 9.39%, O 10.65%
Literature References: Physiological metabolite of vitamin A,
q.v. Effects gene expression via nuclear retinoic acid receptors (RAR); mediates cellular growth and differentiation. Prepn: D. A. van Dorp, J. R. Arens,
Rec. Trav. Chim. 65, 338 (1946); C. D. Robeson
et al., J. Am. Chem. Soc. 77, 4111 (1955). Single-step process: R. Marbet,
DE 2061507;
US 3746730 (1971, 1973 both to Hoffmann-La Roche). Crystal structure: C. H. Stam, C. H. MacGillavry,
Acta Crystallogr. 16, 62 (1963). Toxicology: J. J. Kamm,
J. Am. Acad. Dermatol. 6, 652 (1982). Clinical evaluation in treatment of photoaged skin: J. S. Weiss
et al., J. Am. Med. Assoc. 259, 527 (1988); in promyelocytic leukemia: R. P. Warrell, Jr.
et al., Leukemia 8, 929 (1994). Review of pharmacology and therapeutic potential: G. D. Goss
et al., Crit. Rev. Clin. Lab. Sci. 29, 185-215 (1992). Book:
The Retinoids, M. B. Sporn
et al., Eds. (Raven Press, New York, 2nd ed., 1994) 679 pp.
Properties: Yellow to light orange crystalline powder with characteristic floral odor. Crystals from ethanol, mp 180-182°. uv max (methanol): 351 nm (e 45000). Sol in DMSO; slightly sol in polyethylene glycol 400, octanol, ethanol. Practically insol in water, mineral oil, glycerin. LD50 (10 day) in mice, rats (mg/kg): 790, 790 i.p.; 2200, 2000 orally (Kamm).
Melting point: mp 180-182°
Absorption maximum: uv max (methanol): 351 nm (e 45000)
Toxicity data: LD50 (10 day) in mice, rats (mg/kg): 790, 790 i.p.; 2200, 2000 orally (Kamm)
Derivative Type: 9-
cis-Form
see Alitretinoin
Derivative Type: 13-
cis-Form
see Isotretinoin
Derivative Type: a-Tocopheryl ester
see Tocoretinate
Therap-Cat: Antiacne; adjunct in treatment of photodamaged skin; antineoplastic (hormonal).
Keywords: Antiacne; Keratolytic.